Pancreatic cancer diagnostics play a crucial role in early detection and accurate assessment of the disease. These applications encompass various diagnostic methods, including imaging modalities, biomarker analysis, and genetic testing, enabling precise identification of pancreatic cancer at its initial stages. The features of these diagnostics include advanced imaging technologies, sensitive biomarker detection, and genetic profiling. The advantages lie in timely detection, facilitating prompt treatment, improved patient outcomes, and enhanced survival rates, ultimately leading to better management of pancreatic cancer and improved quality of life for patients.
Access full Report @ https://www.databridgemarketresearch.com/reports/us-pancreatic-cancer-diagnostics-market
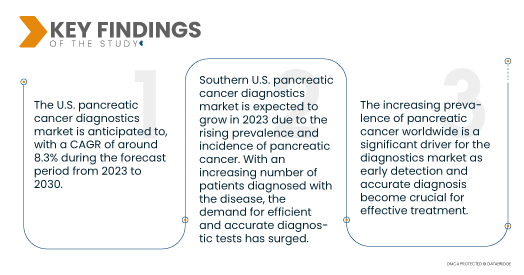
Data Bridge Market Research analyses that the U.S. Pancreatic Cancer Diagnostics Market is registering a CAGR of 8.3% during the forecast period of 2023 to 2030. Continuous developments in diagnostic technologies, such as imaging modalities, biomarker analysis, and genetic testing, provide more accurate and sensitive methods for pancreatic cancer detection, thus driving the market’s growth.
Key Findings of the Study
Growing awareness and screening programs are expected to drive the market's growth rate
As awareness about the significance of early detection and regular screenings for pancreatic cancer grows, more individuals are proactively seeking diagnostic tests. Early detection plays a crucial role in improving treatment outcomes and patient survival rates. Healthcare professionals are emphasizing the importance of timely screenings, leading to increased patient demand for diagnostic tests that can identify pancreatic cancer at its early stages. This rising awareness is driving the healthcare system to prioritize pancreatic cancer diagnostics, ensuring timely interventions and better management of the disease.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Segments Covered
|
Test Type (Imaging Test, Biopsy, Blood Test, Genomic Test, and Others), Cancer Stage (Stage 0, Stage I, Stage II, Stage III, and Stage IV), Tumor Type (Exocrine Tumors and Neuroendocrine Tumors), Product (Instrument-Based Products, Platform-Based Products, Kits and Reagents, and Other Consumables), Technology (Fluorescent In Situ Hybridization, Next Generation Sequencing, Fluoroimmunoassay, Comparative Genomic Hybridization, Immunohistochemical, and Others), Application (Screening, Diagnostic and Predictive, Prognostic, and Research), End User (Hospitals, Diagnostic Centers, Cancer Research Centers, Academic Institutes, Ambulatory Surgical Centers, and Others), Distribution Channel (Direct Tender, Retail Sales, and Others)
|
|
Market Players Covered
|
Agilent Technologies, Inc. (U.S.), BD (U.S.), Biological Dynamics (U.S.), CANON MEDICAL SYSTEMS CORPORATION (U.S.), ClearNote Health (U.S.), CTK Biotech, Inc. (U.S.), Danaher (U.S.), Exact Sciences Corporation (U.S.), F. Hoffman- La Roche Ltd (Switzerland), FUJIFILM Holdings America Corporation (U.S.), GRAIL (U.S.), Immunovia (Sweden), Koninklijke Philips N.V. (Netherlands), Laboratory Corporation of America Holdings (U.S.), MP BIOMEDICALS (U.S.), Myriad Genetics, Inc. (U.S.), QIAGEN (Germany), Quest Diagnostics Incorporated (U.S.), Siemens Healthcare GmbH (Germany), Thermo Fisher Scientific Inc. (U.S.)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework
|
Segment Analysis:
The U.S. pancreatic cancer diagnostics market is segmented on the basis of test type, cancer stages, tumor type, product, application, technology, end user, and distribution channel.
- On the basis of test type, the market is segmented into imaging test, biopsy, blood test, genomic test, and others.
- On the basis of cancer stages, the market is segmented into stage 0, stage I, stage II, stage III, and stage IV.
- On the basis of tumor type, the market is segmented into exocrine tumors and neuroendocrine tumors.
- On the basis of product, the market is segmented into instrument-based products, platform-based products, kits and reagents, and other consumables.
- On the basis of application, the market is segmented into screening, diagnostic and predictive, prognostic, and research.
- On the basis of technology, the market is segmented into fluorescent in situ hybridization, next generation sequencing, fluoroimmunoassay, comparative genomic hybridization, immunohistochemical, and others.
- On the basis of end user, the market is segmented into hospitals, diagnostic centers, cancer research centers, academic institutes, ambulatory surgical centers, and others.
- On the basis of distribution channel, the market is segmented into direct tender, retail sales, and others.
Major Players
Data Bridge Market Research recognizes the following companies as the major U.S. pancreatic cancer diagnostics market players in U.S. pancreatic cancer diagnostics market are Agilent Technologies, Inc. (U.S.), BD (U.S.), Biological Dynamics (U.S.), CANON MEDICAL SYSTEMS CORPORATION (U.S.), ClearNote Health (U.S.), CTK Biotech, Inc. (U.S.), Danaher (U.S.)
Market Developments
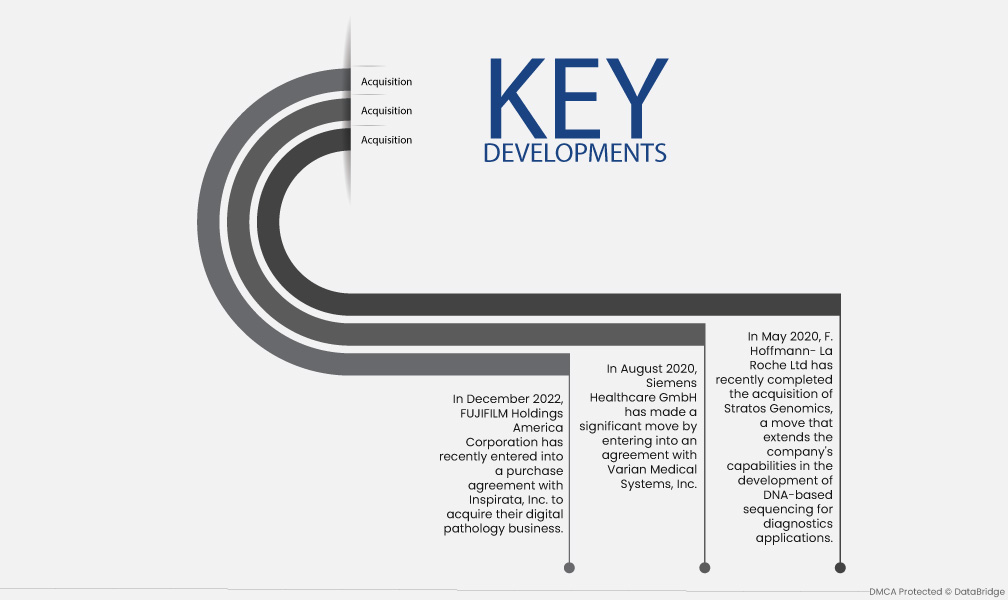
- In December 2022, FUJIFILM Holdings America Corporation has recently entered into a purchase agreement with Inspirata, Inc. to acquire their digital pathology business. This strategic move aims to enhance FUJIFILM's enterprise imaging portfolio by integrating pathology images and data into healthcare organizations' electronic health record systems.
- In August 2020, Siemens Healthcare GmbH has made a significant move by entering into an agreement with Varian Medical Systems, Inc. Through this acquisition, Siemens Healthcare GmbH is strengthening its position in the healthcare industry and contributing to the development of advanced solutions for cancer treatment.
- In May 2020, F. Hoffmann- La Roche Ltd has recently completed the acquisition of Stratos Genomics, a move that extends the company's capabilities in the development of DNA-based sequencing for diagnostics applications. This strategic acquisition strengthens Roche's position in the healthcare diagnosis segment, enabling the company to generate additional revenue through innovative and advanced diagnostic solutions.
Regional Analysis
As per Data Bridge Market Research analysis:
The southern U.S. pancreatic cancer diagnostics market is expected to grow during the forecast period 2023 - 2030
In 2023 Southern U.S. pancreatic cancer diagnostics market is expected to grow due to the increasing prevalence and incidence of pancreatic cancer have driven the demand for efficient and accurate diagnostic tests. Additionally, the growing awareness about the importance of early detection and timely screenings for pancreatic cancer has led to higher patient engagement in seeking diagnostic services. These key factors are expected to contribute significantly to the growth of the pancreatic cancer diagnostics market, as healthcare providers and patients prioritize early identification and management of the disease for improved treatment outcomes and better patient care.
For more detailed information about the pancreatic cancer diagnostics market report, click here – https://www.databridgemarketresearch.com/reports/us-pancreatic-cancer-diagnostics-market