Europe Stroke Diagnostics Market, By Severity (Moderate, Severe, Mild), Type (Computed Tomography (CT Scan), Computed Tomography Angiography (CTA), Magnetic Resonance Imaging (MRI), Magnetic Resonance Angiography (MRA), Transcranial Doppler Ultrasound, Video Head Impulse Test (VHIT), Others), Application (Ischemic Stroke, Hemorrhagic Stroke, Transient Ischemic Attacks (TIAS)), End User (Hospitals, Clinics, Ambulatory Surgical Centers, Home Healthcare), Distribution Channel ( Direct Tender, Third Party Distributors, Others), Stage (Pre Operative, Peri Operative, Post Operative), Country (Germany, France, U.K., Italy, Spain, Russia, Netherland, Switzerland, Turkey, Austria, Belgium, and Rest of Europe), Industry Trends and Forecast to 2028
 Market Analysis and Insights: Europe Stroke Diagnostics Market
Market Analysis and Insights: Europe Stroke Diagnostics Market
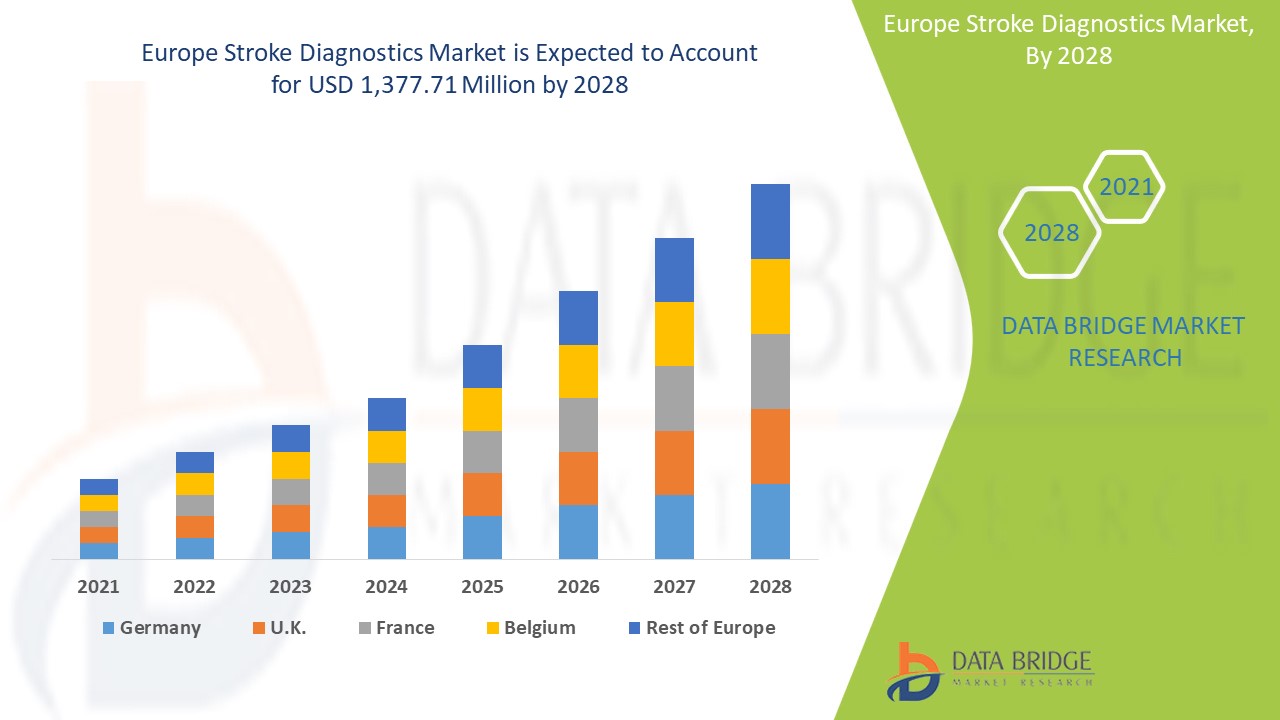
Europe stroke diagnostics market is expected to gain market growth in the forecast period of 2021 to 2028. Data Bridge Market Research analyses that the market is growing with a CAGR of 6.0% in the forecast period of 2021 to 2028 and is expected to reach USD 1,377.71 million by 2028. An increase in products development and rising demand for early diagnosis are the major drivers which propelled the demand of the market in the forecast period. However, stringent regulations may hinder market growth.
A stroke occurs when the supply of blood to the brain is decreased or blocked completely, which prevents the brain tissue from getting oxygen and nutrients. There are various types of diagnostics devices that are being used to detect stroke and its early symptoms, for instance, computed tomography (CT SCAN), computed tomography angiography (CTA), magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), transcranial Doppler ultrasound and others.
The increasing incidence of stroke and cardiovascular and neurological disease has increased the demand for stroke diagnostics in developing countries. Also, the rise in healthcare expenditure is propelling market growth. On the other hand, the high cost of diagnosis may hinder market growth. The presence of market players and new product launches is providing an opportunity. However, the lack of skilled professionals may challenge the market.
The stroke diagnostics market report provides details of market share, new developments, the impact of domestic and localized market players, analysis of opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario, contact us for an Analyst Brief. Our team will help you create a revenue impact solution to achieve your desired goal.
 Europe Stroke Diagnostics Market Scope and Market Size
Europe Stroke Diagnostics Market Scope and Market Size
The Europe stroke diagnostics market is categorized into six notable segments which are based on the severity, type, application, end user, distribution channel and stage. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
- On the basis of severity, the Europe stroke diagnostics market is segmented into moderate, severe, and mild. In 2021, the moderate segment is expected to dominate the market due to an increase in healthcare expenditure and healthcare facilities.
- On the basis of type, the Europe stroke diagnostics market is segmented into computed tomography (CT Scan), computed tomography angiography (CTA), magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), transcranial doppler ultrasound, video head impulse test (VHIT), other. In 2021, the computed tomography (CT scan) segment is expected to dominate the market with the increasing enhancement in technology, which provides accurate results in lesser time.
- On the basis of application, the Europe stroke diagnostics market is segmented into ischemic stroke, hemorrhagic stroke, and transient ischemic attacks (TIAS). In 2021, ischemic stroke is expected to dominate the market with the increasing enhancement in the technology for stroke diagnostics.
- On the basis of end-users, the Europe stroke diagnostics market is segmented into hospitals, home healthcare, ambulatory surgical centers, and clinics. In 2021, the hospital segment is expected to dominate the market with the presence of all the diagnostics devices for diagnosis.
- On the basis of distribution channel, the Europe stroke diagnostics market is segmented into direct tender, third party distributor, and others. In 2021, direct tender is expected to dominate the market due to the presence of various major market players.
- On the basis of stage, the Europe stroke diagnostics market is segmented into pre-operative, peri-operative, and post-operative. In 2021, pre-operative is expected to dominate the market due to the increasing incidence of stroke and cardiovascular disease.
Europe Stroke Diagnostics Market Country Level Analysis
The Europe stroke diagnostics market is analyzed, and market size information is provided into six notable segments, which are severity, type, application, end user, distribution channel, and stage.
The countries covered in the stroke diagnostics market report are the Germany, U.K., France, Italy, Spain, Netherlands, Russia, Switzerland, Belgium, Turkey, Austria, Norway, Hungary, Lithuania, Ireland, Poland, Rest of Europe.
The stroke diagnostics segment in Europe region is expected to grow with the highest growth rate in the forecast period of 2021 to 2028 due to the rising demand for stroke diagnostics. Germany is expected to dominate the Europe stroke diagnostics market due to the growth of R&D in the healthcare industry.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of European brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of sales channels are considered while providing forecast analysis of the country data.
Rising Healthcare Expenditure is Boosting the Market Growth of Stroke Diagnostics
The stroke diagnostics market also provides you with detailed market analysis for every country's growth in the stroke diagnostics industry with stroke diagnostics drugs sales, the impact of advancement in the Stroke diagnostics technology, and changes in regulatory scenarios with their support for the Stroke diagnostics market. The data is available for the historic period 2010 to 2019.
Competitive Landscape and Stroke Diagnostics Market Share Analysis
Stroke diagnostics market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width, and breadth, application dominance, technology lifeline curve. The above data points provided are only related to the company's focus related to the stroke diagnostics market.
The major companies which are dealing in the Europe stroke diagnostics market are Koninklijke Philips N.V., Stryker, Hologic, Inc., ESAOTE SPA, Shimadzu Corporation, Aspect Imaging Ltd, Siemens, Neusoft Corporation, Medfield diagnostics AB, FUJIFILM Holdings Corporation, General Electric Company, ALPINION MEDICAL SYSTEMS Co., Ltd, Shenzhen Mindray Bio-Medical Electronics Co., Ltd, Analogic Corporation, Carestream Health, BPL Medical Technologies, IMRIS, Deerfield Imaging Inc., Canon Inc., SAMSUNG, SternMed GmbH among others.
Many product launches and agreements are also initiated by companies worldwide, which also accelerates the stroke diagnostics market.
For instance,
- In May 2021, Siemens announced that they are launching the new Somatom X.ceed1, a new high-resolution, high-speed CT (computed tomography) scanner engineered specifically for the most challenging clinical areas. It has provided the company with an increase in its product pipeline
- In July 2021, Koninklijke Philips N.V. announced that they are partnering with Cognizant to develop end-to-end digital health solutions that will enable healthcare organizations and life sciences companies to improve patient care and accelerate clinical trials. It will enhance their technological advancement and increases their sales
Collaboration, product launch, business expansion, award and recognition, joint ventures, and other strategies by the market player is enhancing the company market in the Stroke diagnostics market, which also provides the benefit for the organization to improve their offering for Stroke diagnostics.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE STROKE DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 EPIDEMIOLOGY
6 EUROPE STROKE DIAGNOSTICS MARKET: REGULATIONS
6.1 REGULATION IN THE U.S.
6.2 REGULATION IN EUROPE
6.3 REGULATION IN CHINA
6.4 REGULATION IN JAPAN
6.5 REGULATION IN SOUTH AFRICA
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 TECHNOLOGICAL ADVANCEMENT
7.1.2 INCREASE IN THE INCIDENCE OF STROKE
7.1.3 INCREASE IN THE GERIATRIC POPULATION
7.1.4 INCREASE IN NUMBER OF PATIENTS WITH HYPERTENSION AND CORONARY HEART DISEASES
7.2 RESTRAINTS
7.2.1 HIGH COST OF DIAGNOSIS
7.2.2 INCREASE IN PRODUCT RECALL
7.3 OPPORTUNITIES
7.3.1 RISE IN HEALTHCARE SPENDING
7.3.2 INCREASE IN DIABETIC POPULATION AND OBESITY
7.3.3 INCREASE IN FDA APPROVAL AND PRODUCT LAUNCH
7.3.4 RISE IN AWARENESS REGARDING HEALTH AND STROKE
7.4 CHALLENGES
7.4.1 UNFAVORABLE REIMBURSEMENT SCENARIO
7.4.2 COMPLICATION RELATED TO DIAGNOSTIC DEVICES
8 IMPACT OF COVID-19 ON EUROPE STROKE DIAGNOSTICS MARKET
8.1 IMPACT ON PRICE
8.2 IMPACT ON DEMAND
8.3 IMPACT ON SUPPLY CHAIN
8.4 STRATEGIC INITIATIVES
8.5 CONCLUSION
9 EUROPE STROKE DIAGNOSTICS MARKET, BY SEVERITY
9.1 OVERVIEW
9.2 MODERATE
9.3 SEVERE
9.4 MILD
10 EUROPE STROKE DIAGNOSTICS MARKET, BY TYPE
10.1 OVERVIEW
10.2 COMPUTED TOMOGRAPHY (CT SCAN)
10.3 COMPUTED TOMOGRAPHY ANGIOGRAPHY (CTA)
10.4 MAGNETIC RESONANCE IMAGING (MRI)
10.5 MAGNETIC RESONANCE ANGIOGRAPHY (MRA)
10.6 TRANSCRANIAL DOPPLER ULTRASOUND
10.7 VIDEO HEAD IMPULSE TEST (VHIT)
10.8 OTHERS
10.8.1 CAROTID ULTRASOUND
10.8.2 CAROTID ANGIOGRAPHY
10.8.3 ELECTROCARDIOGRAPHY (EKG)
10.8.4 ECHOCARDIOGRAPHY
10.8.5 BLOOD TESTS
10.8.6 NUCLEAR NEUROIMAGING
10.8.7 OTHERS
11 EUROPE STROKE DIAGNOSTICS MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 ISCHEMIC STROKE
11.2.1 THROMBOTIC STROKES
11.2.2 EMBOLIC STROKES
11.3 HEMORRHAGIC STROKE
11.3.1 INTRACEREBRAL HEMORRHAGE
11.3.2 SUBARACHNOID HEMORRHAGE
11.4 TRANSIENT ISCHEMIC ATTACKS (TIAS)
12 EUROPE STROKE DIAGNOSTICS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITAL
12.3 CLINICS
12.4 AMBULATORY SURGICAL CENTERS
12.5 HOME HEALTHCARE
12.6 OTHERS
13 EUROPE STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 THIRD PARTY DISTRIBUTORS
13.4 OTHERS
14 EUROPE STROKE DIAGNOSTICS MARKET, BY STAGE
14.1 OVERVIEW
14.2 PRE OPERATIVE
14.3 PERI OPERATIVE
14.4 POST OPERATIVE
15 EUROPE STROKE DIAGNOSTICS MARKET, BY REGION
15.1 EUROPE
15.1.1 GERMANY
15.1.2 FRANCE
15.1.3 U.K.
15.1.4 ITALY
15.1.5 SPAIN
15.1.6 RUSSIA
15.1.7 NETHERLANDS
15.1.8 SWITZERLAND
15.1.9 TURKEY
15.1.10 AUSTRIA
15.1.11 BELGIUM
15.1.12 REST OF EUROPE
16 STROKE DIAGNOSTICS MARKET COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: EUROPE
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 SIEMENS
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 COMPANY SHARE ANALYSIS
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENTS
18.2 KONINKLIJKE PHILIPS N.V.
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 GENERAL ELECTRIC COMPANY
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENTS
18.4 CANON INC
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENTS
18.5 SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENTS
18.6 ALPINION MEDICAL SYSTEMS CO., LTD
18.6.1 COMPANY SNAPSHOT
18.6.2 PRODUCT PORTFOLIO
18.6.3 RECENT DEVELOPMENTS
18.7 ANALOGIC CORPORATION
18.7.1 COMPANY SNAPSHOT
18.7.2 PRODUCT PORTFOLIO
18.7.3 RECENT DEVELOPMENT
18.8 ASPECT IMAGING LTD
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENT
18.9 BPL MEDICAL TECHNOLOGIES
18.9.1 COMPANY SNAPSHOT
18.9.2 PRODUCT PORTFOLIO
18.9.3 RECENT DEVELOPMENT
18.1 CARESTREAM HEALTH
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PRODUCT PORTFOLIO
18.10.4 RECENT DEVELOPMENTS
18.11 ESAOTE SPA
18.11.1 COMPANY SNAPSHOT
18.11.2 PRODUCT PORTFOLIO
18.11.3 RECENT DEVELOPMENTS
18.12 FONAR CORP
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENT
18.13 FUJIFILM HOLDINGS CORPORATION
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENTS
18.14 HOLOGIC, INC
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PRODUCT PORTFOLIO
18.14.4 RECENT DEVELOPMENTS
18.15 IMRIS, DEERFIELD IMAGING INC.
18.15.1 COMPANY SNAPSHOT
18.15.2 PRODUCT PORTFOLIO
18.15.3 RECENT DEVELOPMENT
18.16 MEDFIELD DIAGNOSTICS AB
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 MEDTRON AG
18.17.1 COMPANY SNAPSHOT
18.17.2 PRODUCT PORTFOLIO
18.17.3 RECENT DEVELOPMENT
18.18 NEUSOFT CORPORATION
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENT
18.19 SAMSUNG
18.19.1 COMPANY SNAPSHOT
18.19.2 REVENUE ANALYSIS
18.19.3 PRODUCT PORTFOLIO
18.19.4 RECENT DEVELOPMENTS
18.2 SHENXHEN ANKE HIGH-TECH CO., LTD.
18.20.1 COMPANY SNAPSHOT
18.20.2 PRODUCT PORTFOLIO
18.20.3 RECENT DEVELOPMENT
18.21 SHIMADZU CORPORATION
18.21.1 COMPANY SNAPSHOT
18.21.2 REVENUE ANALYSIS
18.21.3 PRODUCT PORTFOLIO
18.21.4 RECENT DEVELOPMENTS
18.22 SIUI
18.22.1 COMPANY SNAPSHOT
18.22.2 PRODUCT PORTFOLIO
18.22.3 RECENT DEVELOPMENTS
18.23 STERNMED GMBH
18.23.1 COMPANY SNAPSHOT
18.23.2 PRODUCT PORTFOLIO
18.23.3 RECENT DEVELOPMENT
18.24 STRYKER
18.24.1 COMPANY SNAPSHOT
18.24.2 REVENUE ANALYSIS
18.24.3 PRODUCT PORTFOLIO
18.24.4 RECENT DEVELOPMENTS
18.25 TERASON DIVISION TERATECH CORPORATION
18.25.1 COMPANY SNAPSHOT
18.25.2 PRODUCT PORTFOLIO
18.25.3 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
Lista de Tabela
TABLE 1 EUROPE STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 2 EUROPE MODERATE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 3 EUROPE SEVERE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 4 EUROPE MILD IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 5 EUROPE STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 6 EUROPE COMPUTED TOMOGRAPHY (CT SCAN) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 7 EUROPE COMPUTED TOMOGRAPHY ANGIOGRAPHY (CTA) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 8 EUROPE MAGNETIC RESONANCE IMAGING (MRI) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 9 EUROPE MAGNETIC RESONANCE ANGIOGRAPHY (MRA) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 10 EUROPE TRANSCRANIAL DOPPLER ULTRASOUND IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 11 EUROPE VIDEO HEAD IMPULSE TEST (VHIT) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 12 EUROPE OTHERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 13 EUROPE OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 14 EUROPE STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 15 EUROPE ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 16 EUROPE ISCHEMIC STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 17 EUROPE HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 18 EUROPE HEMORRHAGIC STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 19 EUROPE TRANSIENT ISCHEMIC ATTACKS (TIAS) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 20 EUROPE STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 21 EUROPE HOSPITAL IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 22 EUROPE CLINICS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 23 EUROPE AMBULATORY SURGICAL CENTERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 24 EUROPE HOME HEALTHCARE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 25 EUROPE OTHERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 26 EUROPE STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 27 EUROPE DIRECT TENDER IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 28 EUROPE THIRD PARTY DISTRIBUTORS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 29 EUROPE OTHERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 30 EUROPE STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 31 EUROPE PRE OPERATIVE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 32 EUROPE PERI OPERATIVE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 33 EUROPE POST OPERATIVE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 34 EUROPE STROKE DIAGNOSTICS MARKET, BY COUNTRY, 2019-2028 (USD MILLION)
TABLE 35 EUROPE STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 36 EUROPE STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 37 EUROPE OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 38 EUROPE STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 39 EUROPE ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 40 EUROPE HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 41 EUROPE STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 42 EUROPE STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 43 EUROPE STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 44 GERMANY STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 45 GERMANY STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 46 GERMANY OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 47 GERMANY STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 48 GERMANY ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 49 GERMANY HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 50 GERMANY STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 51 GERMANY STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 52 GERMANY STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 53 FRANCE STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 54 FRANCE STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 55 FRANCE OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 56 FRANCE STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 57 FRANCE ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 58 FRANCE HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 59 FRANCE STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 60 FRANCE STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 61 FRANCE STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 62 U.K. STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 63 U.K. STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 64 U.K. OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 65 U.K. STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 66 U.K. ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 67 U.K. HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 68 U.K. STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 69 U.K. STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 70 U.K. STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 71 ITALY STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 72 ITALY STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 73 ITALY OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 74 ITALY STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 75 ITALY ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 76 ITALY HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 77 ITALY STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 78 ITALY STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 79 ITALY STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 80 SPAIN STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 81 SPAIN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 82 SPAIN OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 83 SPAIN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 84 SPAIN ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 85 SPAIN HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 86 SPAIN STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 87 SPAIN STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 88 SPAIN STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 89 RUSSIA STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 90 RUSSIA STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 91 RUSSIA OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 92 RUSSIA STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 93 RUSSIA ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 94 RUSSIA HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 95 RUSSIA STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 96 RUSSIA STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 97 RUSSIA STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 98 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 99 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 100 NETHERLANDS OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 101 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 102 NETHERLANDS ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 103 NETHERLANDS HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 104 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 105 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 106 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 107 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 108 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 109 SWITZERLAND OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 110 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 111 SWITZERLAND ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 112 SWITZERLAND HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 113 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 114 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 115 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 116 TURKEY STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 117 TURKEY STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 118 TURKEY OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 119 TURKEY STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 120 TURKEY ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 121 TURKEY HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 122 TURKEY STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 123 TURKEY STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 124 TURKEY STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 125 AUSTRIA STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 126 AUSTRIA STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 127 AUSTRIA OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 128 AUSTRIA STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 129 AUSTRIA ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 130 AUSTRIA HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 131 AUSTRIA STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 132 AUSTRIA STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 133 AUSTRIA STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 134 BELGIUM STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 135 BELGIUM STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 136 BELGIUM OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 137 BELGIUM STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 138 BELGIUM ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 139 BELGIUM HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 140 BELGIUM STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 141 BELGIUM STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 142 BELGIUM STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 143 REST OF EUROPE STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
Lista de Figura
FIGURE 1 EUROPE STROKE DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 EUROPE STROKE DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE STROKE DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 EUROPE STROKE DIAGNOSTICS MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE STROKE DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE STROKE DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE STROKE DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE STROKE DIAGNOSTICS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 EUROPE STROKE DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE STROKE DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN DEMAND FOR STROKE DIAGNOSTISIS EXPECTED TO DRIVE THE EUROPE STROKE DIAGNOSTICS MARKET IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 12 MODERATE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE STROKE DIAGNOSTICS MARKET IN 2021 & 2028
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE STROKE DIAGNOSTICS MARKET, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE STROKE DIAGNOSTICS MARKET
FIGURE 15 EUROPE STROKE DIAGNOSTICS MARKET: BY SEVERITY, 2020
FIGURE 16 EUROPE STROKE DIAGNOSTICS MARKET: BY SEVERITY, 2020-2028 (USD MILLION)
FIGURE 17 EUROPE STROKE DIAGNOSTICS MARKET: BY SEVERITY, CAGR (2021-2028)
FIGURE 18 EUROPE STROKE DIAGNOSTICS MARKET: BY SEVERITY, LIFELINE CURVE
FIGURE 19 EUROPE STROKE DIAGNOSTICS MARKET: BY TYPE, 2020
FIGURE 20 EUROPE STROKE DIAGNOSTICS MARKET: BY TYPE, 2020-2028 (USD MILLION)
FIGURE 21 EUROPE STROKE DIAGNOSTICS MARKET: BY TYPE, CAGR (2021-2028)
FIGURE 22 EUROPE STROKE DIAGNOSTICS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 23 EUROPE STROKE DIAGNOSTICS MARKET: BY APPLICATION, 2020
FIGURE 24 EUROPE STROKE DIAGNOSTICS MARKET: BY APPLICATION, 2020-2028 (USD MILLION)
FIGURE 25 EUROPE STROKE DIAGNOSTICS MARKET: BY APPLICATION, CAGR (2021-2028)
FIGURE 26 EUROPE STROKE DIAGNOSTICS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 27 EUROPE STROKE DIAGNOSTICS MARKET: BY END USER, 2020
FIGURE 28 EUROPE STROKE DIAGNOSTICS MARKET: BY END USER, 2020-2028 (USD MILLION)
FIGURE 29 EUROPE STROKE DIAGNOSTICS MARKET: BY END USER, CAGR (2021-2028)
FIGURE 30 EUROPE STROKE DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 EUROPE STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2020
FIGURE 32 EUROPE STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2020-2028 (USD MILLION)
FIGURE 33 EUROPE STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2021-2028)
FIGURE 34 EUROPE STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 35 EUROPE STROKE DIAGNOSTICS MARKET: BY STAGE, 2020
FIGURE 36 EUROPE STROKE DIAGNOSTICS MARKET: BY STAGE, 2020-2028 (USD MILLION)
FIGURE 37 EUROPE STROKE DIAGNOSTICS MARKET: BY STAGE, CAGR (2021-2028)
FIGURE 38 EUROPE STROKE DIAGNOSTICS MARKET: BY STAGE, LIFELINE CURVE
FIGURE 39 EUROPE STROKE DIAGNOSTICS MARKET: SNAPSHOT (2020)
FIGURE 40 EUROPE STROKE DIAGNOSTICS MARKET: BY COUNTRY (2020)
FIGURE 41 EUROPE STROKE DIAGNOSTICS MARKET: BY COUNTRY (2021 & 2028)
FIGURE 42 EUROPE STROKE DIAGNOSTICS MARKET: BY COUNTRY (2020 & 2028)
FIGURE 43 EUROPE STROKE DIAGNOSTICS MARKET: BY SEVERITY (2021-2028)
FIGURE 44 STROKE DIAGNOSTICS MARKET COMPANY SHARE 2020 (%)
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.













