Global Primary Biliary Cholangitis Therapeutics Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
1.03 Billion
USD
2.01 Billion
2024
2032
USD
1.03 Billion
USD
2.01 Billion
2024
2032
| 2025 –2032 | |
| USD 1.03 Billion | |
| USD 2.01 Billion | |
|
|
|
Global Primary Biliary Cholangitis Therapeutics Market Segmentation, By Drug type (Primary Drug and Secondary Drug), Distribution Channel (Hospital Pharmacies, Drug Store & Retail Pharmacies, and Online Pharmacies) – Industry Trends and Forecast to 2032
Primary Biliary Cholangitis Therapeutics Market Analysis
The primary biliary cholangitis therapeutics market is driven by the increasing prevalence of the disease, a growing focus on early diagnosis, and advancements in treatment options. The market has seen significant growth in recent years due to the introduction of novel therapies targeting the underlying mechanisms of PBC. Drugs such as obeticholic acid and ursodeoxycholic acid (UDCA) have gained traction as first-line treatments, although their limitations in efficacy have fueled the demand for more effective alternatives.
Biologics and immune-modulating therapies are expected to play a significant role in the market, with numerous clinical trials underway for drugs that can offer better control over the disease's progression and liver function. The market is also witnessing the rise of combination therapies, which are believed to provide enhanced outcomes by targeting multiple pathways involved in PBC pathogenesis.
The increasing awareness about PBC and the potential for targeted therapies are expected to drive the growth of the market. In addition, key players in the pharmaceutical industry are investing in research and development, aiming to bring new treatments to market. As the disease burden rises and therapeutic options improve, the PBC therapeutics market is anticipated to expand steadily in the coming years.
Primary Biliary Cholangitis Therapeutics Market Size
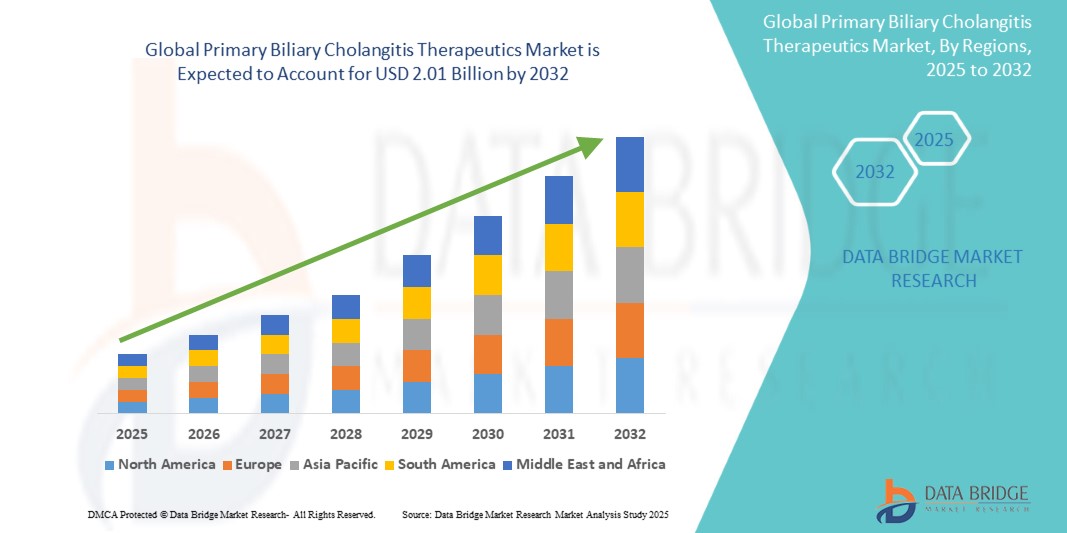
The global primary biliary cholangitis therapeutics market size was valued at USD 1.03 billion in 2024 and is projected to reach USD 2.01 billion by 2032, with a CAGR of 8.72% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Primary Biliary Cholangitis Therapeutics Market Trends
“Increasing Focus on Combination Therapies”
One notable trend in the primary biliary cholangitis therapeutics market is the increasing focus on combination therapies. As single-agent treatments such as ursodeoxycholic acid (UDCA) have shown limited efficacy in some patients, researchers and pharmaceutical companies are turning to combination therapies that target multiple disease pathways simultaneously. These therapies often combine traditional treatments with newer biologics or immune-modulating drugs to improve disease management and slow progression.
This trend is driven by the growing understanding of PBC’s complex pathophysiology, which involves immune system dysfunction and liver damage. By targeting various aspects of the disease, combination therapies aim to provide better clinical outcomes, reduce the risk of liver complications, and potentially improve long-term survival rates. Ongoing clinical trials are evaluating the safety and efficacy of these combinations, signalling a shift toward personalized and more effective treatments for PBC patients, ultimately reshaping the future of the therapeutics market.
Report Scope and Primary Biliary Cholangitis Therapeutics Market Segmentation
|
Attributes |
Primary Biliary Cholangitis Therapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
ADVANZ PHARMA (England), Alkem (India), AbbVie Inc. (U.S.), Calliditas Therapeutics AB (Sweden), COUR Pharmaceuticals (U.S.), Cadila Pharmaceuticals (India), Gilead Sciences, Inc. (U.S.), GSK plc. (U.K.), GENFIT (France), Ipsen Pharma (France), ICE S.p.a (Italy), Intercept Pharmaceuticals, Inc. (U.S.), Ironwood (U.S.), Lupin (India), Lilly (U.S.), Leeford Healthcare Ltd (India), Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co.,Ltd. (China), Sun Pharmaceutical Industries Ltd. (India), Viatris Inc. (U.S.) and Zydus Therapeutics Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Primary Biliary Cholangitis Therapeutics Market Definition
Primary biliary cholangitis therapeutics refer to the medications and treatments used to manage and alleviate the symptoms of PBC, a chronic autoimmune liver disease that causes progressive damage to the bile ducts. These therapies aim to slow disease progression, improve liver function, and manage complications. Common treatments include ursodeoxycholic acid (UDCA), which is often the first-line therapy, and newer agents such as obeticholic acid and elafibranor, which are used for patients who do not respond adequately to UDCA. The goal of PBC therapeutics is to reduce inflammation, prevent liver damage, and in some cases, manage coexisting conditions or complications related to the disease.
Primary Biliary Cholangitis Therapeutics Market Dynamics
Drivers
- Rising Prevalence of Primary Biliary Cholangitis (PBC)
The increasing global prevalence of primary biliary cholangitis is a major driver for the growth of the PBC therapeutics market. Primary Biliary Cholangitis primarily affects middle-aged women, leading to a growing patient population that demands effective treatment. As awareness about the disease improves, more people are diagnosed early, prompting the need for new and better therapeutic options. This rise in patient numbers is accelerating the demand for advanced treatments, such as obeticholic acid and setanaxib, which offer improved efficacy compared to older therapies such as ursodeoxycholic acid (UDCA). The growing prevalence of PBC, coupled with an increase in early diagnosis, contributes to a larger market for PBC therapeutics. This trend is expected to sustain the market growth as pharmaceutical companies continue to develop and release targeted therapies to meet the rising demand.
- Advancements in Treatment Options
The ongoing advancements in the development of novel therapies for PBC are significantly driving market growth. Over the past few years, the approval of new drugs such as obeticholic acid (Ocaliva) and - (Iqirvo) has expanded the treatment landscape for PBC, offering patients more effective options beyond traditional UDCA. In addition, the focus on biologics and immune-modulating treatments has led to more targeted therapies aimed at addressing the root causes of PBC, improving disease management and slowing progression. These innovations have opened up new opportunities for pharmaceutical companies, with combination therapies showing promising results. As these treatments become available to a wider patient base, they are expected to boost the PBC therapeutics market significantly, improving patient outcomes and driving continued growth in the sector.
Opportunities
- Expansion of Combination Therapies
One significant opportunity in the primary biliary cholangitis therapeutics market lies in the development and expansion of combination therapies. Many patients with PBC do not respond adequately to single-agent treatments such as ursodeoxycholic acid (UDCA), driving the demand for therapies that combine different drug classes for enhanced efficacy. For instance, the combination of obeticholic acid (Ocaliva) with UDCA has shown promise in improving treatment outcomes for patients with inadequate response to UDCA alone. As more combination therapies enter the market, they are expected to improve disease management by addressing multiple disease pathways simultaneously. This will likely boost patient outcomes and increase market demand for combination treatment options, ultimately accelerating the growth of the PBC therapeutics market.
- Development of Targeted and Personalized Therapies
The growing focus on personalized medicine presents a significant opportunity for the PBC therapeutics market. Researchers are increasingly exploring targeted therapies that cater to specific patient profiles, taking into account genetic and molecular factors. For instance, drugs such as elafibranor (Iqirvo) are designed to target specific inflammatory pathways involved in PBC, offering more tailored treatment options for patients who may not respond to traditional therapies. As personalized therapies gain more traction, they hold the potential to improve treatment efficacy and reduce adverse effects, leading to better overall patient outcomes. This shift towards precision medicine in PBC is expected to fuel the market’s growth, as healthcare providers and pharmaceutical companies focus on more individualized treatment strategies.
Restraints/Challenges
- Regulatory Challenges and Delayed Approvals
One key restraint in the Primary Biliary Cholangitis therapeutics market is the regulatory hurdles and delayed approvals of new treatments. The lengthy and complex approval process for new drugs can slow the introduction of innovative therapies, limiting treatment options for patients with PBC. For instance, despite promising results in clinical trials, drugs such as obeticholic acid and elafibranor faced extended evaluation periods before gaining approval in certain regions. These delays can hinder market growth by prolonging the time it takes for patients to access effective therapies. In addition, regulatory requirements for conducting extensive trials and meeting safety standards can further complicate and delay the development of new treatments. These regulatory challenges may reduce the overall market potential and limit timely access to therapies, affecting both patients and pharmaceutical companies aiming to enter the market.
- Limited Awareness and Early Diagnosis
A significant challenge facing the PBC therapeutics market is the limited awareness and early diagnosis of the disease. PBC is often diagnosed in its later stages, which can lead to severe liver damage and complications. The disease is rare and typically progresses slowly, causing delays in diagnosis, which limits the effectiveness of interventions. Limited awareness among both healthcare providers and patients about PBC contributes to misdiagnosis or late-stage detection, making treatment less effective. While increased awareness and early diagnosis are critical for improving patient outcomes, addressing this challenge requires ongoing education and improved screening methods. Overcoming this challenge would accelerate the adoption of therapies and drive market growth, as earlier intervention leads to better treatment outcomes and more effective management of the disease.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Primary Biliary Cholangitis Therapeutics Market Scope
The market is segmented on the basis of drug type and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug type
- Primary Drug
- Type
- Obeticholic Acid
- Ursodeoxycholic Acid
- Others
- Secondary Drug
Distribution Channel
- Hospital Pharmacies, Drug Store and Retail Pharmacies
- Online Pharmacies
Primary Biliary Cholangitis Therapeutics Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, drug type, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the Primary Biliary Cholangitis therapeutics market due to the high prevalence of PBC, advanced healthcare infrastructure, and the presence of key pharmaceutical companies. The region benefits from increased awareness, early diagnosis, and access to innovative treatments such as obeticholic acid and elafibranor. In addition, favorable reimbursement policies and a large number of ongoing clinical trials contribute to the market's growth in North America, making it a leading region in the global PBC therapeutics market.
Asia-Pacific is expected to exhibit the highest growth rate in the Primary Biliary Cholangitis therapeutics market. The region is witnessing an increase in the diagnosis of PBC, driven by improved healthcare infrastructure, greater awareness, and rising healthcare spending. With an aging population and growing demand for advanced treatments, countries such as China and Japan are expected to contribute significantly to market expansion. The increasing availability of innovative therapies and a larger patient base will drive rapid market growth in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Primary Biliary Cholangitis Therapeutics Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Primary Biliary Cholangitis Therapeutics Market Leaders Operating in the Market Are:
- ADVANZ PHARMA (England)
- Alkem (India)
- AbbVie Inc. (U.S.)
- Calliditas Therapeutics AB (Sweden)
- COUR Pharmaceuticals (U.S.)
- Cadila Pharmaceuticals (India)
- Gilead Sciences, Inc. (U.S.)
- GSK plc. (U.K.)
- GENFIT (France)
- Ipsen Pharma (France)
- ICE S.p.a (Italy)
- Intercept Pharmaceuticals, Inc. (U.S.)
- Ironwood (U.S.)
- Lupin (India)
- Lilly (U.S.)
- Leeford Healthcare Ltd (India)
- Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co.,Ltd. (China)
- Sun Pharmaceutical Industries Ltd. (India)
- Viatris Inc. (U.S.)
- Zydus Therapeutics Inc. (U.S.)
Latest Developments in Primary Biliary Cholangitis Therapeutics Market
- In February 2025, Gilead Sciences, Inc. announced that the European Commission (EC) has granted conditional marketing authorization for seladelpar. This treatment is approved for use in combination with ursodeoxycholic acid (UDCA) in adults with primary biliary cholangitis (PBC) who have an insufficient response to UDCA alone, or as a monotherapy for those who cannot tolerate UDCA
- In November 2024, Intercept Pharmaceuticals, Inc., a wholly owned biopharmaceutical subsidiary of Alfasigma S.p.A., announced that the U.S. Food and Drug Administration (FDA) has issued a Complete Response Letter (CRL) regarding the supplemental New Drug Application (sNDA) for OCALIVA (obeticholic acid, OCA). The sNDA was seeking full approval for the treatment of patients with primary biliary cholangitis (PBC), a rare, progressive disease that predominantly affects women
- In August 2024, Gilead Sciences, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval for Livdelzi (seladelpar) to treat primary biliary cholangitis (PBC). The approval covers its use in combination with ursodeoxycholic acid (UDCA) for adults who have not responded adequately to UDCA, or as a monotherapy for patients who are unable to tolerate UDCA
- In July 2024, Calliditas Therapeutics AB announced that the Phase 2b TRANSFORM trial achieved its primary endpoint, demonstrating a statistically significant reduction in ALP (Alkaline Phosphatase) for both tested doses compared to placebo. The trial assessed setanaxib, a NOX enzyme inhibitor, in patients with primary biliary cholangitis (PBC) and elevated liver stiffness
- In June 2024, Ipsen announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval for Iqirvo (elafibranor) 80 mg tablets for the treatment of primary biliary cholangitis (PBC). The approval allows Iqirvo to be used in combination with ursodeoxycholic acid (UDCA) for adults who have had an insufficient response to UDCA, or as a monotherapy for those unable to tolerate UDCA. Iqirvo is now available for prescription to eligible patients in the U.S.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.