Asia Pacific Acute Lymphocytic Lymphoblastic Leukemia All Diagnostics Market
Market Size in USD Million
CAGR :
% 
 USD
208.08 Million
USD
388.02 Million
2024
2032
USD
208.08 Million
USD
388.02 Million
2024
2032
| 2025 –2032 | |
| USD 208.08 Million | |
| USD 388.02 Million | |
|
|
|
|
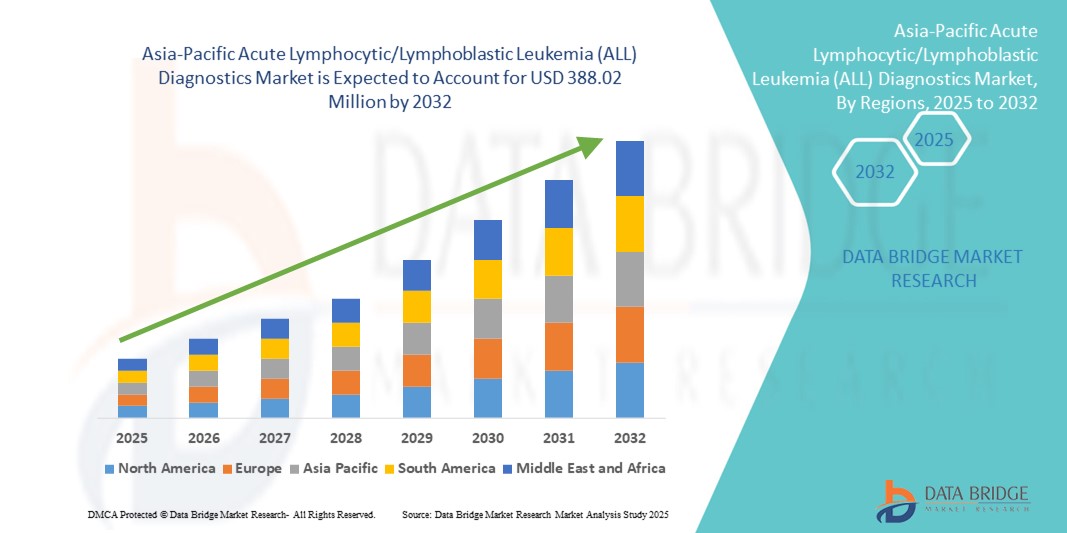
Asia-Pacific Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics Market Size
- The Asia-Pacific Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics market size was valued at USD 208.08 million in 2024 and is expected to reach USD 388.02 million by 2032, at a CAGR of 8.10% during the forecast period
- The market growth is primarily driven by the rising prevalence of Acute lymphocytic/lymphoblastic leukemia (ALL) across the Asia-Pacific region, leading to an increasing demand for accurate and early diagnostic solutions. Advancements in diagnostic technologies, such as next-generation sequencing (NGS), flow cytometry, and molecular diagnostics, are enabling healthcare providers to detect ALL with higher precision and speed. In addition, growing awareness about leukemia screening and the availability of advanced diagnostic tools in both urban and semi-urban healthcare settings are further supporting market expansion
- Moreover, significant investments in oncology-focused laboratory infrastructure, the establishment of specialized hematology and oncology diagnostic centers, and the expansion of cancer testing facilities in emerging economies such as India, China, and Indonesia are bolstering the adoption of ALL diagnostics. Supportive government initiatives for cancer detection, along with increasing collaborations between global diagnostic companies and local healthcare providers, are contributing to improved accessibility, affordability, and innovation in the Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market
Asia-Pacific Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics Market Analysis
- The Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market is witnessing significant growth, driven by rising prevalence of leukemia, advancements in molecular and genetic testing technologies, and increasing healthcare investments across countries such as China, India, Japan, South Korea, Australia, Thailand, Indonesia, and Vietnam
- Expansion of specialized oncology laboratories, growing adoption of precision medicine, increasing availability of targeted therapies, and rising awareness programs for early leukemia detection are accelerating market penetration across the region
- China dominated the Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market, accounting for the largest revenue share of 43.2% in 2024, supported by its strong diagnostic infrastructure, large patient pool, growing integration of next-generation sequencing (NGS) in routine diagnostics, and government initiatives to improve cancer care accessibility
- India is projected to register the fastest CAGR of 19.4% during the forecast period, driven by rapid expansion of oncology-focused diagnostic chains, increased participation in global clinical trials, rising demand for cost-effective testing solutions, and penetration of molecular diagnostics into tier-2 and tier-3 cities
- The B-cell lymphoblastic leukemia/lymphoma segment held a dominant position with a substantial market share of 68.3%. This leadership is primarily attributed to its higher incidence rate across the Asia-Pacific region, which has prompted increased research focus and significant advancements in diagnostic technologies specifically targeting this subtype
Report Scope and Asia-Pacific Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics Market Segmentation
|
Attributes |
Asia-Pacific Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Asia-Pacific Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics Market Trends
Advancements in Precision Diagnostics and Regulatory Compliance Driving Market Growth
- A key trend in the Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market is the rising emphasis on precision diagnostics and stringent regulatory compliance. Efforts are focused on improving the accuracy of ALL detection, speeding up diagnostic turnaround times, and adhering to international quality and safety standards tailored for hematologic cancers
- Leading diagnostic companies and laboratories in the region are collaborating with pharmaceutical and biotechnology firms to deploy advanced diagnostic techniques such as flow cytometry, molecular genetic profiling, and next-generation sequencing (NGS). These technologies provide highly reliable and validated diagnostic results critical for effective disease management and personalized treatment plans
- Increasing integration of ALL diagnostics in clinical and research settings is driving market expansion, supporting early disease detection, treatment efficacy monitoring, and ensuring adherence to regulatory mandates across healthcare providers in Asia-Pacific
- Research institutions, universities, and government labs in countries including India, Japan, China, and Australia are actively developing novel diagnostic assays, automating laboratory workflows, and validating new testing methodologies. These initiatives enhance diagnostic precision and optimize testing efficiency in ALL care
- With the Asia-Pacific region’s growing focus on healthcare innovation, regulatory rigor, and improving patient outcomes, the Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics market is expected to witness continuous growth, propelled by technological advancements, stronger compliance frameworks, and enhanced collaboration between research and diagnostic laboratories
Asia-Pacific Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics Market Dynamics
Driver
Rising Demand Fueled by Healthcare Expansion and Technological Advancements
- The Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market is growing rapidly, supported by expanding healthcare infrastructure, pharmaceutical advancements, and biotechnology research across key countries such as China, India, Japan, South Korea, and Australia. Increasing investments in drug discovery, biosimilar development, and personalized medicine are driving demand for high-quality ALL diagnostic services compliant with international standards
- For instance, in March 2024, WuXi AppTec enhanced its analytical testing facilities in China, expanding bioanalytical and diagnostic capabilities to help domestic and global clients accelerate regulatory approvals
- A rising incidence of hematological cancers and the emphasis on early and precise diagnosis are encouraging healthcare providers to adopt advanced ALL diagnostic technologies. Government initiatives promoting local research and clinical trials further accelerate market growth
- Singapore and South Korea are becoming regional hubs for diagnostic innovation, attracting global clients through strong intellectual property laws and competitive pricing
- The adoption of digital diagnostic platforms and Laboratory Information Management Systems (LIMS) is improving test accuracy, turnaround times, and compliance with regulatory frameworks across the region
Restraint/Challenge
Challenges Limiting Growth in Rural and Small-Scale Healthcare Settings
- Despite robust growth in metropolitan and urban centers, the Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market faces significant challenges when it comes to penetrating rural areas and smaller healthcare facilities, particularly in developing regions of Southeast Asia and South Asia. One of the primary hurdles is the high cost associated with advanced diagnostic testing. Many small-scale healthcare providers and clinics operate on limited budgets and are unable to afford sophisticated diagnostic equipment or outsourced laboratory services. This financial barrier restricts access to timely and accurate ALL diagnostics for a substantial portion of the population residing in rural and semi-urban regions
- In addition to cost issues, a lack of awareness about the benefits and availability of modern ALL diagnostic techniques hampers adoption. Healthcare practitioners and patients in remote areas often rely on traditional or basic testing methods, which may not detect ALL at an early stage or with the precision necessary for effective treatment planning. This gap in knowledge and education results in delayed diagnoses and poorer health outcomes
- Infrastructure limitations also pose a major challenge. Many rural and remote regions suffer from inadequate transportation and logistics networks, making it difficult to collect and transport biological samples safely and promptly to centralized testing laboratories. This leads to longer turnaround times for diagnostic results, which can negatively impact patient management and treatment decisions. In addition, cold chain facilities and proper storage conditions for samples may be lacking, further compromising test accuracy
- The scarcity and uneven distribution of accredited and well-equipped diagnostic laboratories exacerbate these problems. Patients and healthcare providers in remote locations often have to rely on distant urban centers or international facilities for complex ALL diagnostic tests. This geographic disparity increases operational costs, delays care, and places additional strain on patients who must travel long distances for testing
Asia-Pacific Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics Market Scope
The market is segmented on the basis of product type, test type, cancer type, age group, gender, end user, and distribution channel.
- By Product Type
On the basis of product type, the Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market is segmented into instruments and consumables & accessories. In 2024, the instruments segment dominated the market with a substantial revenue share of 62.4%. This dominance is primarily driven by the widespread use of sophisticated diagnostic instruments, including flow cytometers, PCR machines, and next-generation sequencing platforms, which are essential for the accurate and early detection of Acute Lymphocytic/Lymphoblastic Leukemia (ALL). These advanced instruments play a crucial role in monitoring disease progression and enabling personalized treatment approaches.
Conversely, the consumables & accessories segment is projected to register the fastest CAGR of 9.2% between 2025 and 2032, fueled by rising demand for critical reagents, diagnostic kits, and disposable laboratory supplies that support high-throughput testing and ensure precise diagnostic results.
- By Test Type
On the basis of test type, the Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market is segmented into imaging test, biopsy, blood test, and others. The blood test segment led the market in 2024 with a revenue share of 45.6%, largely due to its minimally invasive procedure, cost-effectiveness, and broad applicability across screening, initial diagnosis, and ongoing monitoring of disease progression in ALL patients.
Meanwhile, the biopsy segment is expected to achieve the fastest growth with a CAGR of 10.1% over the forecast period, reflecting its indispensable role in confirming ALL diagnoses, providing detailed pathological insights, and facilitating the development of tailored treatment plans for patients.
- By Cancer Type
On the basis of cancer type, the Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market is segmented into B-cell lymphoblastic leukemia/lymphoma and T-cell lymphoblastic leukemia. In 2024, the B-cell lymphoblastic leukemia/lymphoma segment held a dominated position with a substantial market share of 68.3%. This leadership is primarily attributed to its higher incidence rate across the Asia-Pacific region, which has prompted increased research focus and significant advancements in diagnostic technologies specifically targeting this subtype.
Meanwhile, the T-cell lymphoblastic leukemia segment, although less common, is anticipated to grow steadily at a CAGR of 7.4% between 2025 and 2032. This growth is supported by continuous improvements in immunophenotyping techniques and molecular diagnostics, enabling more accurate detection and tailored treatment approaches for this clinically important but relatively rarer leukemia subtype.
- By Age Group
On the basis of age group, the Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market is segmented into Below 21, 21-29, 30-65, and 65 and above. The Below 21 age group accounted for the largest revenue share of 38.7% in 2024, reflecting the well-documented higher prevalence of acute lymphoblastic leukemia (ALL) among children and adolescents within the Asia-Pacific region. This high prevalence drives demand for pediatric-specific diagnostic solutions and early intervention therapies.
Meanwhile, the 30-65 age group is projected to show robust market growth with a CAGR of 8.3% over the forecast period. This growth is largely driven by rising awareness of ALL symptoms in adults, improved diagnostic capabilities that facilitate earlier detection, and a growing focus on adult patient populations that were historically underdiagnosed.
- By Gender
On the basis of gender, the Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market is segmented into male and female. The male segment dominated the market in 2024, holding a revenue share of 53.2%. This predominance aligns with epidemiological data indicating a marginally higher incidence of ALL in males across the region. Factors such as genetic predisposition and environmental influences are being studied to understand this gender disparity better.
Conversely, the Female segment is expected to experience steady growth with a CAGR of 7.9% throughout the forecast period. This trend is reflective of improvements in healthcare access, increased health awareness among women, and advancements in diagnostic technologies that facilitate earlier and more accurate detection of ALL in female patients.
- By End User
On the basis of end user, the Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market is segmented into hospitals, associated labs, independent diagnostic laboratories, diagnostic imaging centers, cancer research institutes, and others. In 2024, hospitals dominated the market with a significant share of 57.6%, largely due to their well-established and advanced diagnostic infrastructure, ability to provide integrated patient care, and capacity to manage complex and severe leukemia cases efficiently.
Meanwhile, independent diagnostic laboratories and cancer research institutes are poised to experience the fastest growth, registering CAGRs of 9.5% and 9.2%, respectively. This rapid expansion is driven by the increasing trend of outsourcing diagnostic services by healthcare providers and a surge in investments directed towards research and clinical trials focused on leukemia diagnostics, enhancing capabilities and service reach in these specialized settings.
- By Distribution Channel
On the basis of distribution channel, the Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market is segmented into direct tender and retail sales. Direct tender held the largest market share of 54.3% in 2024, supported primarily by bulk procurement activities conducted by government healthcare agencies and large hospital networks aiming to fulfill the escalating demand for leukemia diagnostic solutions.
On the other hand, the retail sales segment is anticipated to witness the fastest CAGR of 10.3% during the forecast period from 2025 to 2032. This rapid growth is propelled by the expansion of online sales platforms, an increasing footprint in smaller clinics, and improved accessibility of diagnostic products and services in semi-urban and rural areas, enabling wider reach and convenience for end users.
Asia-Pacific Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics Market Regional Analysis
- Asia-Pacific held a market share of 32,1% in the global acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market in 2024
- This strong market position is underpinned by the region’s extensive diagnostic infrastructure, a large patient population, and the growing integration of next-generation sequencing (NGS) technologies into routine diagnostic workflows. In addition, government initiatives aimed at improving cancer care accessibility and expanding early detection programs have significantly bolstered the region’s capabilities in ALL diagnostics
- The presence of advanced laboratories and increasing collaboration between healthcare providers and technology innovators further fuel market expansion across countries such as China, India, Japan, and South Korea
China Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics Market Insight
The China acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market led the Asia-Pacific market with a dominant revenue share of 43.2% in 2024. This leadership is supported by its robust diagnostic infrastructure, large patient pool, and proactive government programs focused on enhancing cancer diagnosis and treatment. The country is at the forefront of adopting cutting-edge technologies such as NGS and molecular profiling, which improve diagnostic accuracy and enable personalized treatment approaches. Moreover, China’s well-established network of hospitals and specialized cancer centers, combined with strong R&D investment, strengthens its market dominance. Rapid urbanization and increased healthcare spending continue to drive demand for sophisticated ALL diagnostic services, positioning China as the key growth engine within the region.
India Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics Market Insight
The India acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics market is projected to register the fastest CAGR of 19.4% during the forecast period, fueled by the rapid expansion of oncology-focused diagnostic chains and increased participation in global clinical trials. The rising demand for affordable and accessible testing solutions, coupled with the growing penetration of molecular diagnostics into tier-2 and tier-3 cities, is accelerating market growth. Government initiatives promoting healthcare infrastructure development and digital health adoption are also enhancing diagnostic reach and quality. In addition, collaborations between Indian diagnostic laboratories and international research organizations are boosting the availability of advanced ALL testing technologies. As a result, India is emerging as one of the fastest-growing markets for ALL diagnostics in the Asia-Pacific region.
Asia-Pacific Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics Market Share
The Asia-Pacific acute lymphocytic/lymphoblastic leukemia (ALL) diagnostics industry is primarily led by well-established companies, including:
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Thermo Fisher Scientific, Inc. (U.S.)
- QIAGEN (Netherlands)
- Abbott (U.S.)
- Merck KGaA (Germany)
- Siemens Healthineers AG (Germany)
- Hologic, Inc. (U.S.)
- Agilent Technologies, Inc. (U.S.)
- DiaSorin S.p.A. (Italy)
- Illumina, Inc. (U.S.)
- Myriad Genetics, Inc. (U.S.)
- BIOMÉRIEUX (France)
- Quest Diagnostics Incorporated (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- BD (U.S.)
- Exact Sciences Corporation (U.S.)
- Time Medical Holding (China)
- PlexBio (China)
- MinFound Medical Systems Co., Ltd (China)
- Medonica Co. LTD (Israel)
Latest Developments in Asia-Pacific Acute Lymphocytic/Lymphoblastic Leukemia (ALL) Diagnostics market
- In August 2022, F. Hoffmann-La Roche Ltd. launched the Digital LightCycler System, a next-generation digital PCR (dPCR) platform designed to provide extremely sensitive and absolute quantification of DNA/RNA targets. Although Roche described the system for global oncology and infectious-disease testing, the platform’s ultra-sensitivity and absolute quantification capability directly enable improved detection of low-frequency leukemia variants and MRD (minimal residual disease) — features highly relevant to ALL diagnostics labs across Asia-Pacific that require sensitive MRD workflows
- In November 2023, Roche announced the LightCycler PRO qPCR system, positioning it as a next-generation real-time PCR solution for clinical diagnostics and research that supports higher throughput and automation. The availability of advanced qPCR platforms like LightCycler PRO boosted molecular testing capacity in APAC clinical labs for oncology panels, including assays used in leukemia diagnostics and monitoring
- In February 2023, Thermo Fisher Scientific launched a Cell Therapy Collaboration Centre Programme in Singapore, establishing a regional hub to accelerate cell and gene therapy development across APAC. While the programme principally supports therapeutic development and manufacturing, Thermo Fisher’s deeper footprint in Singapore (and related clinical/molecular offerings across APAC) has had ancillary impacts: local hospitals and labs gained greater access to high-throughput genomic platforms, reagents and local support — resources that are frequently leveraged for advanced hematologic diagnostics such as ALL molecular profiling and MRD testing
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.