Asia Pacific Anti Nuclear Antibody Test Market
Market Size in USD Million
CAGR :
% 
 USD
558.77 Million
USD
1,639.25 Million
2024
2032
USD
558.77 Million
USD
1,639.25 Million
2024
2032
| 2025 –2032 | |
| USD 558.77 Million | |
| USD 1,639.25 Million | |
|
|
|
|
Asia-Pacific Anti-Nuclear Antibody Test Market Size
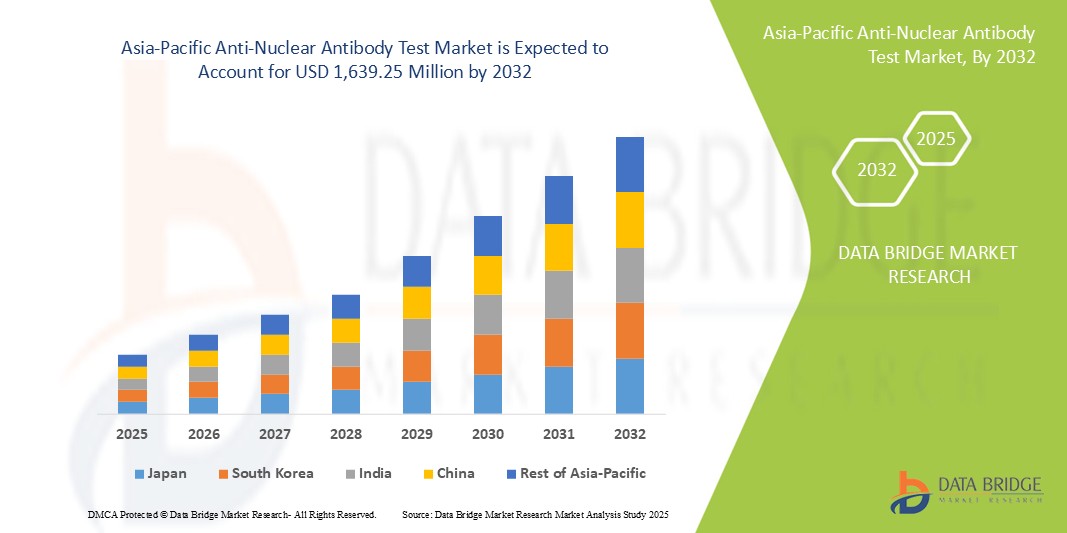
- The Asia-Pacific anti-nuclear antibody test market size was valued at USD 558.77 million in 2024 and is expected to reach USD 1,639.25 million by 2032, at a CAGR of 14.40% during the forecast period
- This growth is driven by factors such as the rising prevalence of autoimmune diseases, technological advancements in diagnostics, and increasing healthcare awareness
Asia-Pacific Anti-Nuclear Antibody Test Market Analysis
- Anti-Nuclear Antibody (ANA) tests are critical diagnostic tools used to detect autoantibodies in the blood, aiding in the diagnosis of autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and Sjögren’s syndrome. These tests play a vital role in early detection and management of these conditions
- The growing incidence of autoimmune disorders, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis, is propelling the demand for ANA testing across the region
- China is expected to dominate the Asia-Pacific anti-nuclear antibody test market with 32.4% market share, driven by its large population base, increasing prevalence of autoimmune diseases, and significant investments in healthcare infrastructure
- India is expected to be the fastest growing country with a CAGR of 14.6% in the Asia-Pacific anti-nuclear antibody test market driven by rising healthcare awareness and increasing healthcare spending
- ELISA (Enzyme-Linked Immunosorbent Assay) is expected to dominate the market with a market share of 52.60% due to its high sensitivity, cost-effectiveness, and widespread availability, making it the preferred choice for large-scale screening in clinical laboratories
Report Scope and Asia-Pacific Anti-Nuclear Antibody Test Market Segmentation
|
Attributes |
Asia-Pacific Anti-Nuclear Antibody Test Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Asia-Pacific Anti-Nuclear Antibody Test Market Trends
“Advancements in Anti-Nuclear Antibody (ANA) Testing & Automation for Autoimmune Diagnostics in Asia-Pacific”
- One notable trend in the evolution of ANA testing in the Asia-Pacific region is the increasing use of automated testing platforms and enhanced diagnostic technologies
- These innovations significantly improve the speed, accuracy, and efficiency of ANA testing, providing higher sensitivity and reliability in detecting autoimmune disorders
- For instance, automated immunofluorescence assay (IFA) systems are streamlining the testing process, enabling labs to handle larger volumes of samples with reduced human error and faster turnaround times, which is crucial for accurate autoimmune disease diagnosis
- The rise in the development and adoption of point-of-care testing devices further contributes to the market's growth. These devices provide quicker results, facilitating timely treatment decisions, especially in remote areas with limited access to specialized healthcare
- These advancements are transforming autoimmune diagnostics in Asia-Pacific, improving the accuracy of diagnosis and enhancing early detection of autoimmune diseases, thereby driving the demand for more efficient and accessible ANA testing solutions
Asia-Pacific Anti-Nuclear Antibody Test Market Dynamics
Driver
“Growing Prevalence of Autoimmune Diseases”
- The increasing prevalence of autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, and autoimmune thyroid disorders is significantly driving the demand for ANA testing in the Asia-Pacific region
- As the population in many Asia-Pacific countries ages and environmental factors contribute to immune system changes, there is a notable rise in the incidence of autoimmune diseases, particularly among women
- With more individuals being diagnosed with these conditions, the demand for ANA tests is increasing, ensuring accurate diagnosis and better disease management outcomes
For instance,
- In October 2023, the World Health Organization (WHO) reported that the prevalence of autoimmune diseases in Asia is increasing, especially in countries such as China and India, which have large aging populations and growing healthcare awareness
- As a result, the rising incidence of autoimmune diseases has created a pressing need for ANA testing, thereby expanding the market for diagnostic tools
Opportunity
“Technological Advancements in ANA Testing”
- The development of automated, high-throughput diagnostic platforms for ANA testing offers a significant opportunity to increase testing efficiency and accuracy
- Automation can reduce the risk of human error, increase throughput in laboratories, and speed up the diagnostic process, making it more accessible to larger populations, especially in rural or underserved regions
- In addition, the adoption of point-of-care (POC) testing devices for ANA testing presents an opportunity to improve diagnosis in locations with limited access to specialized laboratories
For instance,
- In March 2024, a study published in the Asian Journal of Clinical Immunology highlighted that automated IFA (Immunofluorescence Assay) systems are becoming increasingly popular in countries such as Japan and South Korea, improving the overall testing accuracy and efficiency in busy clinical settings
- By leveraging automation and POC technology, ANA tests can be performed more rapidly, providing quicker diagnostic results and enabling timely medical interventions for autoimmune diseases
Restraint/Challenge
“High Cost and Limited Access to Diagnostic Infrastructure”
- The cost of ANA testing, along with the limited availability of advanced diagnostic equipment in rural or developing areas, poses a challenge to the growth of the market in the Asia-Pacific region
- Although ANA tests are crucial for diagnosing autoimmune diseases, the expense of maintaining advanced diagnostic systems can be a barrier for healthcare providers in low-resource settings
- In addition, the need for highly skilled laboratory technicians and the complex nature of ANA testing limit access to these diagnostic services in certain regions.
For instance,
- In August 2023, an article published by the Indian Medical Association emphasized the challenge of affordability and infrastructure, particularly in rural parts of India, where access to advanced diagnostic services is limited due to both financial constraints and a lack of trained professionals
- As a result, healthcare systems in these regions may struggle to provide timely and accurate ANA testing, hindering overall market growth in the region
Asia-Pacific Anti-Nuclear Antibody Test Market Scope
The market is segmented on the basis of antibody type, product, technique, application, end user and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Antibody Type |
|
|
By Product |
|
|
By Technique |
|
|
By Application
|
|
|
By End User |
|
|
By Distribution Channel |
|
In 2025, the ELISA is projected to dominate the market with a largest share in technique segment
The ELISA (Enzyme-Linked Immunosorbent Assay) segment is expected to dominate the Asia-Pacific anti-nuclear antibody test market with the largest share of 52.60% in 2025. This dominance is primarily due to its high sensitivity, cost-effectiveness, and widespread availability, making it a preferred choice for large-scale screening in clinical laboratories. The reliability and efficiency of ELISA in detecting autoimmune disorders further contribute to its market leadership.
The extractable nuclear antigens (ENA) is expected to account for the largest share during the forecast period in antibody type market
In 2025, the extractable nuclear antigens (ENA) segment is expected to dominate the market with the largest share of 29.35%. This dominance is primarily due to the critical role of ENA tests in diagnosing specific autoimmune diseases such as systemic lupus erythematosus (SLE) and Sjögren's syndrome. The accuracy and specificity of ENA testing make it a vital component of autoimmune diagnostics, driving its market leadership.
Asia-Pacific Anti-Nuclear Antibody Test Market Regional Analysis
- China holds a dominant share of the Asia-Pacific ANA test market with 32.4% market share, driven by its large population base, increasing prevalence of autoimmune diseases, and significant investments in healthcare infrastructure
- Japan is a leading country in the Asia-Pacific region, known for its advanced healthcare technologies, high healthcare spending, and strong focus on early disease detection. Japan contributes about 20% to the regional ANA test market, reflecting its well-established healthcare system and aging population
- India is projected to experience the highest compound annual growth rate (CAGR) in the market with a market share of 14.6%, driven by rising healthcare awareness and increasing healthcare spending
- Southeast Asian countries, including Indonesia, Malaysia, and the Philippines, are experiencing rapid market growth due to increasing healthcare investments, rising awareness of autoimmune diseases, and expanding diagnostic capabilities
- Australia and New Zealand are also witnessing steady market growth, supported by high healthcare standards, widespread insurance coverage, and a growing elderly population
- The growing trend of point-of-care (POC) diagnostics in Asia-Pacific, particularly in remote and rural regions, is boosting the demand for ANA testing. POC diagnostic solutions in the region
Asia-Pacific Anti-Nuclear Antibody Test
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Thermo Fisher Scientific Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Abbott (U.S.)
- Euroimmun Medizinische Labordiagnostika AG (Germany)
- Revvity Inc. (U.S.)
- Trinity Biotech (Ireland)
- LIFESPAN BIOSCIENCES, INC (U.S.)
- ORIGENE TECHNOLOGIES, INC. (U.S.)
- Abnova Corporation (Taiwan)
- CUSABIO TECHNOLOGY LLC (U.S.)
- Biorbyt Ltd. (England)
Latest Developments in Asia-Pacific Anti-Nuclear Antibody Test Market
- In March 2025, Shenzhen Mindray Bio-Medical Electronics Co., Ltd. announced the launch of its next-generation ANA testing platform in the Asia-Pacific region, designed to improve test accuracy and throughput. The new system integrates advanced automation and artificial intelligence to provide rapid, reliable results for autoimmune disease diagnosis, supporting faster clinical decision-making
- In February 2025, Sysmex Corporation, a leading Japanese diagnostics company, unveiled its enhanced ELISA platform for ANA testing, featuring increased sensitivity and precision. This development aims to address the growing demand for accurate autoimmune diagnostics in the Asia-Pacific market, driven by rising awareness of autoimmune diseases and expanding healthcare infrastructure
- In January 2025, Bio-Rad Laboratories, Inc. expanded its ANA testing portfolio in the Asia-Pacific region with the introduction of a new multiplex assay system, offering comprehensive autoantibody profiling for more precise disease diagnosis. This launch aligns with the company’s strategy to strengthen its market presence in the rapidly growing Asia-Pacific diagnostic sector
- In December 2024, MBL (Medical & Biological Laboratories Co., Ltd.) introduced its latest line of diagnostic reagents for ANA testing in the Asia-Pacific market. These reagents are designed to provide highly specific and accurate results, supporting early diagnosis and effective disease management for autoimmune disorders such as systemic lupus erythematosus (SLE) and rheumatoid arthritis
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.