Asia Pacific Drug Safety Solutions And Pharmacovigilance Market
Market Size in USD Billion
CAGR :
% 
 USD
1.42 Billion
USD
2.65 Billion
2024
2032
USD
1.42 Billion
USD
2.65 Billion
2024
2032
| 2025 –2032 | |
| USD 1.42 Billion | |
| USD 2.65 Billion | |
|
|
|
|
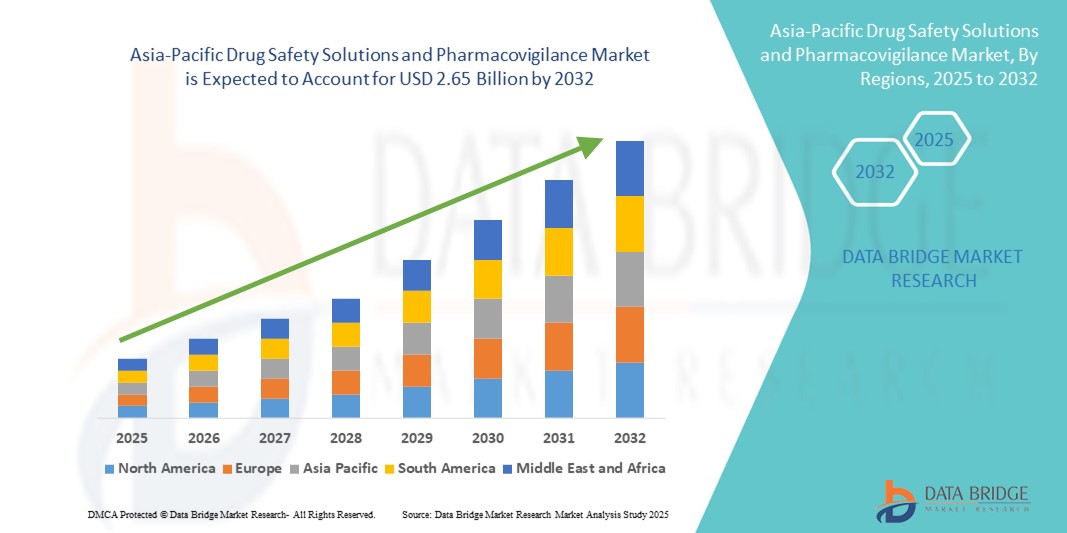
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Size
- The Asia-Pacific drug safety solutions and pharmacovigilance market size was valued at USD 1.42 billion in 2024 and is expected to reach USD 2.65 billion by 2032, at a CAGR of 8.10% during the forecast period
- The market growth is largely fueled by the increasing pharmaceutical R&D activities, stricter regulatory requirements for adverse event reporting, and the expanding adoption of advanced safety monitoring tools across the region’s healthcare and life sciences sectors
- Furthermore, rising demand for real-time safety data analysis, coupled with growing investments in AI-enabled pharmacovigilance platforms, is positioning these solutions as critical components for ensuring drug efficacy and patient safety. These converging factors are accelerating market adoption, thereby significantly boosting the industry's growth
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Analysis
- Drug safety solutions and pharmacovigilance systems, enabling the detection, assessment, and prevention of adverse drug reactions, are becoming essential components of the pharmaceutical and biotechnology sectors in the Asia-Pacific region due to stringent regulatory requirements, the expansion of drug development activities, and the rising need for real-time safety monitoring
- The escalating demand for these solutions is primarily fueled by the surge in clinical trial activity, the increasing prevalence of chronic diseases requiring long-term medication, and a growing emphasis on compliance with international drug safety standards among healthcare providers and manufacturers
- China dominated the Asia-Pacific drug safety solutions and pharmacovigilance market with the largest revenue share of 36.9% in 2024, supported by its rapidly expanding pharmaceutical manufacturing base, large patient population, significant government investment in healthcare digitalization, and the fast adoption of AI-powered pharmacovigilance platforms, with notable growth in both in-house and outsourced safety monitoring services
- India is expected to be the fastest-growing Asia-Pacific drug safety solutions and pharmacovigilance market during the forecast period due to rising R&D investments, modernizing regulatory frameworks, and the increasing use of cloud-based safety data management systems
- The software segment dominated the Asia-Pacific drug safety solutions and pharmacovigilance market with a share of 49.2% in 2024, driven by their scalability, automation features, and seamless integration with existing healthcare IT infrastructure for efficient adverse event data processing
Report Scope and Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Segmentation
|
Attributes |
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Trends
AI-Driven Automation and Cloud-Based Safety Platforms
- A significant and accelerating trend in the Asia-Pacific drug safety solutions and pharmacovigilance market is the growing adoption of artificial intelligence (AI) and advanced analytics within cloud-based platforms to streamline adverse event detection, reporting, and compliance. This integration is enhancing the speed, accuracy, and scalability of safety operations across the region
- For instance, Oracle Argus Safety and ArisGlobal’s LifeSphere Safety platforms are being deployed in several Asia-Pacific countries, offering AI-enabled case processing, automated signal detection, and multilingual adverse event reporting capabilities tailored to local regulatory requirements
- AI integration enables predictive safety insights by analyzing large volumes of structured and unstructured data from clinical trials, electronic health records, and patient registries. For instance, some solutions use machine learning algorithms to identify emerging safety signals before they escalate into major public health issues, improving proactive decision-making
- Cloud deployment allows geographically dispersed teams, including contract research organizations (CROs) and pharmacovigilance outsourcing partners, to collaborate seamlessly, while ensuring secure data sharing and real-time regulatory submissions to authorities such as China’s NMPA or India’s CDSCO
- This trend towards intelligent, automated, and interconnected safety management systems is reshaping the pharmacovigilance landscape in Asia-Pacific, prompting leading providers such as IQVIA and Cognizant to expand AI-enabled service offerings with embedded compliance tools
- The demand for platforms combining AI, automation, and cloud accessibility is rising rapidly as pharmaceutical companies, biotech firms, and regulatory bodies across Asia-Pacific prioritize patient safety and operational efficiency
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Dynamics
Driver
Rising Regulatory Mandates and Expanding Clinical Trials
- The increasingly stringent drug safety regulations across Asia-Pacific, coupled with a surge in clinical trial activity, are driving demand for advanced pharmacovigilance solutions
- For instance, in February 2024, China’s National Medical Products Administration (NMPA) announced updated post-marketing drug safety monitoring guidelines, mandating faster adverse event reporting and integration with centralized safety databases. Such measures are pushing pharmaceutical companies to upgrade their safety infrastructure
- The growing number of chronic disease treatments, biologics, and personalized medicines entering the market further amplifies the need for efficient adverse event management, as these therapies often require long-term safety monitoring
- In addition, global pharmaceutical companies expanding their R&D presence in Asia-Pacific are increasingly partnering with local CROs and adopting region-specific safety systems to ensure compliance and market access
- The combination of regulatory enforcement, growing patient populations, and expanding trial networks is making drug safety systems indispensable for maintaining both compliance and competitive advantage
Restraint/Challenge
Data Privacy Regulations and Skilled Workforce Shortage
- Complex and varying data privacy laws across Asia-Pacific such as China’s Personal Information Protection Law (PIPL) and India’s Digital Personal Data Protection Act pose significant challenges for cross-border pharmacovigilance data management. These regulations often require localization of data storage and strict consent protocols, adding operational complexity
- For example, multinational firms must adapt their safety databases and workflows to meet each country’s data governance rules, which can slow implementation and increase compliance costs
- Furthermore, a shortage of trained pharmacovigilance professionals in emerging Asia-Pacific markets limits the speed at which organizations can scale advanced safety operations. While outsourcing to specialized service providers is helping bridge the gap, the demand for skilled analysts and safety physicians continues to outpace supply
- Deploying AI-enabled pharmacovigilance platforms and cloud-based safety solutions often requires significant upfront investment in software, hardware, and integration with existing IT infrastructure. Smaller pharmaceutical companies or emerging biotech firms may face budget constraints, limiting widespread adoption
- Overcoming these challenges will require strategic investment in workforce training, harmonization of regional data standards, and the deployment of flexible technology platforms capable of meeting both global and local compliance requirements
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Scope
The market is segmented on the basis of type, product, functionality, delivery mode, end users, and distribution channel.
- By Type
On the basis of type, the Asia-Pacific drug safety solutions and pharmacovigilance market is segmented into software and services. The software segment dominated the market with the largest revenue share of 49.2% in 2024, driven by the increasing adoption of AI-enabled automation, real-time adverse event reporting, predictive analytics, and seamless integration with existing healthcare IT infrastructure. Software solutions are preferred by pharmaceutical companies and CROs for their ability to streamline pharmacovigilance processes, ensure compliance with regulatory frameworks, and provide comprehensive dashboards for monitoring safety data across multiple trials and post-marketing studies.
The services segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by the rising trend of outsourcing pharmacovigilance activities to specialized service providers, which enables companies to reduce operational costs, access highly skilled safety professionals, and comply efficiently with local and international regulatory requirements. Services include case processing, medical review, signal detection, risk management, and regulatory submission support, which are increasingly sought after by companies expanding operations in Asia-Pacific.
- By Product
On the basis of product, the Asia-Pacific drug safety solutions and pharmacovigilance market is segmented into standard form and customized form solutions. The standard form segment dominated the market in 2024 due to its ready-to-deploy nature, adherence to regulatory guidelines, and suitability for small- and medium-sized pharmaceutical firms that require quick implementation. Standard solutions offer pre-configured workflows, templates for adverse event reporting, and compliance with international standards such as ICH-GCP and local regulatory bodies, reducing the time and cost required for deployment.
The customized solutions segment is expected to grow at the fastest rate during the forecast period, driven by large pharmaceutical companies and biotech firms that require tailor-made workflows, integration with legacy systems, advanced reporting formats, and specific functionality aligned to unique clinical trial designs or therapeutic areas. Customized platforms offer flexibility, enhanced data visualization, and specialized reporting for multi-country regulatory submissions, catering to complex pharmacovigilance needs.
- By Functionality
On the basis of functionality, the Asia-Pacific drug safety solutions and pharmacovigilance market is segmented into adverse event reporting software, drug safety audit software, and issue tracking software. The adverse event reporting software segment dominated the market in 2024, owing to its critical role in regulatory compliance, ensuring timely reporting of adverse events during clinical trials and post-marketing surveillance. This software helps companies standardize case intake, triage, assessment, and reporting while minimizing human error.
The drug safety audit and issue tracking software segments are expected to witness the fastest growth, driven by increasing regulatory scrutiny, the need for proactive risk identification, and quality assurance in drug development and commercialization. These solutions enable continuous monitoring of compliance status, identification of gaps in safety processes, and structured tracking of follow-up actions, thereby enhancing patient safety and reducing regulatory risk.
- By Delivery Mode
On the basis of delivery, the Asia-Pacific drug safety solutions and pharmacovigilance market is segmented into on-premise and on-demand/cloud-based (SaaS) delivery modes. The on-demand/cloud-based segment dominated in 2024, supported by the growing adoption of cloud infrastructure for scalable, secure, and cost-effective pharmacovigilance operations. Cloud platforms enable real-time collaboration among geographically dispersed teams, facilitate faster regulatory submissions, and reduce the need for in-house IT maintenance and infrastructure investment.
The on-premise delivery mode is expected to grow at the fastest rate during forecast period, particularly in organizations and regions with stringent data privacy and localization requirements. On-premise solutions offer higher control over sensitive safety data, allow for internal customization, and support compliance with local laws, making them suitable for large pharmaceutical companies and multinational firms operating across multiple jurisdictions.
- By End Users
On the basis of end users, the Asia-Pacific drug safety solutions and pharmacovigilance market is segmented into biotechnology and pharmaceuticals, contract research organizations (CROs), hospitals, KPOs/BPOs, and healthcare providers. The biotechnology and pharmaceuticals segment dominated the market in 2024, due to the high volume of clinical trials, extensive post-marketing surveillance, and strict regulatory compliance obligations that necessitate robust safety monitoring systems.
The CROs and hospitals segment is expected to witness the fastest growth, as these organizations increasingly adopt pharmacovigilance solutions to manage outsourced clinical safety monitoring, support multi-center trials, and implement hospital-based adverse event reporting systems. The rising demand for specialized services, combined with expansion of clinical research activities across Asia-Pacific, drives adoption among these end users.
- By Distribution Channel
On the basis of distribution channel, the Asia-Pacific drug safety solutions and pharmacovigilance market is segmented into direct sales and retail sales. The direct sales segment dominated in 2024, driven by long-term contracts with pharmaceutical companies, CROs, and healthcare organizations, ensuring continuous support, software updates, training, and compliance assistance.
The retail sales segment is expected to grow at the fastest rate, particularly for standardized software packages and cloud-based solutions, as smaller firms increasingly procure ready-to-deploy products via online marketplaces or authorized distributors. Retail channels provide cost-effective and faster access to pharmacovigilance tools, enabling broader adoption among emerging biotech companies and small healthcare providers.
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Regional Analysis
- China dominated the Asia-Pacific drug safety solutions and pharmacovigilance market with the largest revenue share of 36.9% in 2024, supported by its rapidly expanding pharmaceutical manufacturing base, large patient population, significant government investment in healthcare digitalization
- Pharmaceutical companies and healthcare organizations in the region highly value real-time safety monitoring, automated adverse event reporting, and seamless integration of software solutions with existing IT infrastructure. This enables faster regulatory submissions, proactive risk management, and improved patient safety outcomes
- The widespread adoption is further supported by government initiatives promoting digital health infrastructure, increased R&D investments, and rising awareness of drug safety requirements. These factors collectively establish pharmacovigilance solutions as critical tools for biotechnology and pharmaceutical companies, CROs, hospitals, and other healthcare providers across Asia-Pacific.
The China Drug Safety Solutions Market Insight
The China drug safety solutions and pharmacovigilance market captured the largest revenue share of 36.9% in 2024 within Asia-Pacific, fueled by the country’s rapidly expanding pharmaceutical manufacturing sector, large patient population, and growing clinical trial activities. Increasing regulatory requirements from the National Medical Products Administration (NMPA) and the adoption of AI-powered pharmacovigilance platforms are driving market growth. Pharmaceutical companies and CROs are prioritizing real-time adverse event reporting, automated safety monitoring, and cloud-based solutions to ensure compliance and improve patient safety outcomes.
Japan Drug Safety Solutions Market Insight
The Japan drug safety solutions and pharmacovigilance market is gaining momentum due to advanced healthcare infrastructure, a focus on regulatory compliance, and high adoption of digital health technologies. Companies in Japan are increasingly using AI-enabled and cloud-based pharmacovigilance platforms to manage clinical trials and post-marketing surveillance. The emphasis on patient safety, coupled with stringent reporting requirements, is driving adoption in biotechnology, pharmaceutical firms, and hospitals.
India Drug Safety Solutions Market Insight
The India drug safety solutions and pharmacovigilance market accounted for one of the fastest-growing revenue shares in Asia-Pacific in 2024, driven by rising clinical trial activities, expansion of the pharmaceutical sector, and government initiatives promoting digital healthcare systems. Increasing outsourcing of pharmacovigilance services to CROs and KPOs, alongside growing adoption of cost-effective cloud-based solutions, is boosting the market. The presence of a large pool of skilled professionals and the push toward smart, data-driven safety monitoring solutions further enhance market growth.
Australia Drug Safety Solutions Market Insight
The Australia drug safety solutions and pharmacovigilance market is witnessing steady growth, supported by the expansion of biotechnology and pharmaceutical research activities, growing focus on post-marketing surveillance, and compliance with TGA (Therapeutic Goods Administration) guidelines. Cloud-based safety platforms and services are increasingly adopted by hospitals, CROs, and healthcare providers to streamline adverse event reporting and enhance patient safety monitoring.
South Korea Drug Safety Solutions Market Insight
The South Korea drug safety solutions and pharmacovigilance market is expected to grow at a significant CAGR, fueled by technological adoption, strong R&D investments, and government emphasis on patient safety. Integration of AI and automation in pharmacovigilance workflows, alongside regulatory mandates for electronic reporting of adverse events, is driving adoption across pharmaceutical companies and CROs.
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Share
The Asia-Pacific drug safety solutions and pharmacovigilance industry is primarily led by well-established companies, including:
- Clinquest Group B.V. (Netherlands)
- Take Solutions Limited (India)
- Wipro Limited (India)
- Fosun Pharma (China)
- Sinopharm (China)
- Sinovac Biotech (China)
- ALTEOGEN Inc. (South Korea)
- A.N.B. Laboratories Co., Ltd. (South Korea)
- Biopharm Chemicals Co., Ltd. (Thailand)
- Boehringer Ingelheim International GmbH (Germany)
- Accenture (Ireland)
- Bristol-Myers Squibb Company (U.S.)
- Labcorp (U.S.)
- ICON plc (Ireland)
- Parexel International (MA) Corporation (U.S.)
- United BioSource LLC (U.S.)
- Cognizant (U.S.)
What are the Recent Developments in Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market?
- In August 2025, Selta Square adopted Oracle Argus Safety, a comprehensive safety platform, to automate and manage pharmacovigilance processes. This implementation is expected to improve compliance, risk evaluation, and scalability for pharmaceutical clients across the Asia-Pacific region
- In July 2025, EVERSANA unveiled EVERSANA ORCHESTRATE PV, an AI-driven solution designed to streamline drug safety workflows. This platform leverages artificial intelligence to enhance the speed, accuracy, and quality of pharmacovigilance processes, aiming to shape the future of drug safety monitoring in the Asia-Pacific region
- In July 2025, Southern Star Research implemented Oracle Argus Safety to strengthen its drug safety processes. This adoption reflects the company's commitment to enhancing clinical capabilities and ensuring reliable and compliant safety and medical monitoring systems as study volumes increase
- In May 2025, the Therapeutic Goods Administration (TGA) of Australia sought public feedback on proposed international clinical trial and pharmacovigilance guidelines. This initiative reflects Australia's commitment to aligning its regulatory practices with global standards, ensuring the safety and efficacy of therapeutic goods. The TGA's efforts aim to enhance collaboration and harmonization in clinical trials and post-market surveillance across the Asia-Pacific region
- In July 2021, Australia's Therapeutic Goods Administration (TGA) released its International Engagement Strategy 2021-2025. The strategy focuses on working with international regulatory counterparts to create a more globally aligned regulatory framework. The TGA's priorities include global policy alignment, pre- and post-market global monitoring, and enhancing regional regulatory capabilities
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.