Asia Pacific Electronic Clinical Outcome Assessment Ecoa Market
Market Size in USD million
CAGR :
% 
 USD
265.11 million
USD
924.58 million
2024
2032
USD
265.11 million
USD
924.58 million
2024
2032
| 2025 –2032 | |
| USD 265.11 million | |
| USD 924.58 million | |
|
|
|
|
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) Market Size
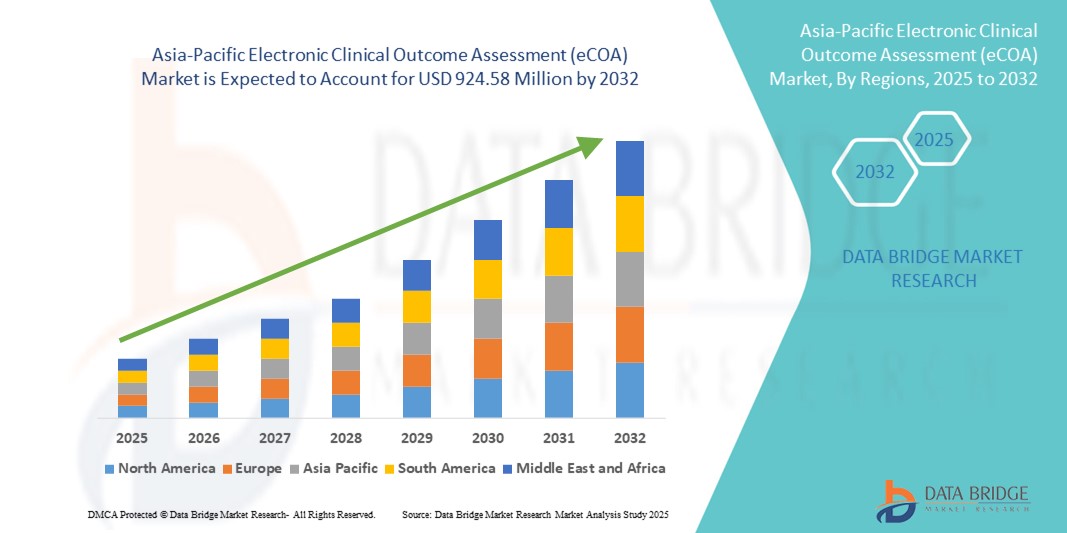
- The Asia-Pacific electronic clinical outcome assessment (eCOA) market size was valued at USD 265.11 million in 2024 and is expected to reach USD 924.58 million by 2032, at a CAGR of 16.90% during the forecast period
- The market growth is primarily driven by increasing adoption of digital health technologies, regulatory encouragement for electronic data capture in clinical trials, and the growing demand for patient-centric and remote monitoring solutions across the region
- In addition, rising focus on improving data accuracy, compliance, and real-time patient insights is positioning eCOA platforms as a vital component in modern clinical research. These factors are collectively accelerating the adoption of eCOA solutions, thereby significantly propelling the market’s expansion in Asia-Pacific
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) Market Analysis
- eCOA platforms, providing electronic capture of patient-reported, clinician-reported, and observer-reported outcomes in clinical trials, are becoming essential tools in modern drug development and clinical research due to their enhanced data accuracy, real-time monitoring capabilities, and seamless integration with other digital health solutions
- The rising adoption of eCOA solutions is primarily driven by increasing digitalization in clinical trials, regulatory encouragement for electronic data collection, and the growing need for patient-centric, remote, and decentralized trial designs
- China dominated the Asia-Pacific electronic clinical outcome assessment (eCOA) market with the largest revenue share of 39% in 2024, characterized by increasing clinical trial activities, rapid digital health adoption, and the presence of key industry players, with significant uptake of eCOA solutions in multicenter and decentralized clinical trials
- India is expected to be the fastest-growing country in the Asia-Pacific electronic clinical outcome assessment (eCOA) market during the forecast period due to rising clinical research investments, government support for digital health initiatives, and growing adoption of patient-centric and mobile-based eCOA solutions
- Patient Reported Outcome Assessment (PRO) segment dominated the Asia-Pacific electronic clinical outcome assessment (eCOA) market with a market share of 42.8% in 2024, driven by its critical role in capturing real-world patient experiences, improving trial compliance, and enhancing overall data reliability
Report Scope and Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) Market Segmentation
|
Attributes |
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) Market Trends
Digital Transformation and Mobile-Based eCOA Solutions
- A significant and accelerating trend in the Asia-Pacific eCOA market is the growing adoption of mobile-based and cloud-enabled platforms that allow real-time patient data capture in clinical trials, improving accuracy and efficiency
- For instance, in 2024, CRF Health (now Medidata Solutions) expanded its mobile eCOA offerings across China and India, enabling patients to report outcomes via smartphones and tablets, increasing trial compliance and data reliability
- Integration with AI and analytics tools is enhancing the functionality of eCOA platforms, enabling predictive insights, automated data quality checks, and early identification of protocol deviations. Some platforms can analyze patient-reported outcomes in real-time to flag inconsistencies or unusual patterns, improving overall trial oversight
- The interoperability of eCOA systems with electronic health records (EHRs) and other clinical trial management systems (CTMS) allows centralized management of trial data, facilitating more efficient monitoring, faster decision-making, and reduced administrative burden
- This trend towards more intelligent, patient-centric, and connected eCOA solutions is transforming clinical trial execution in Asia-Pacific, prompting providers such as Signant Health and Oracle Health Sciences to enhance mobile, cloud, and AI-enabled functionalities for local trial operations
- The demand for mobile-friendly, integrated, and analytics-driven eCOA platforms is rapidly increasing across both pharmaceutical and CRO-led clinical trials, as sponsors and investigators prioritize data accuracy, patient compliance, and operational efficiency
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) Market Dynamics
Driver
Rising Clinical Trial Activity and Digital Health Adoption
- The increasing number of clinical trials in Asia-Pacific, along with rapid adoption of digital health solutions, is a major driver for eCOA market growth
- For instance, in March 2024, PPD announced deployment of eCOA platforms for decentralized clinical trials in India, enabling remote patient monitoring and data collection. Such initiatives by key companies are expected to accelerate market expansion during the forecast period
- As pharmaceutical companies focus on patient-centric trials, eCOA solutions offer benefits such as real-time data capture, automated compliance reminders, and reduced site visits, enhancing both trial quality and participant engagement
- Furthermore, government initiatives supporting digital health and regulatory guidance encouraging electronic patient-reported outcome collection are promoting the adoption of eCOA platforms
- The flexibility of mobile and tablet-based reporting, combined with integration into broader clinical trial management workflows, is making eCOA an indispensable tool in both local and multinational trials across Asia-Pacific
Restraint/Challenge
Regulatory Variations and Data Privacy Concerns
- The differences in regulatory frameworks and data privacy laws across Asia-Pacific countries pose a significant challenge to the widespread adoption of eCOA solutions. Compliance with local data storage, patient consent, and cross-border data transfer regulations requires careful planning and investment
- For instance, trials in China and South Korea must adhere to strict data localization requirements, which can complicate the deployment of cloud-based eCOA systems
- Ensuring data security through robust encryption, secure authentication protocols, and adherence to local regulations is crucial for building trust among sponsors, CROs, and participants. Companies such as Medidata and CRF Health emphasize regulatory compliance and advanced security measures in their Asia-Pacific offerings to mitigate these concerns
- In addition, infrastructure limitations in some regions, such as inconsistent internet connectivity or low digital literacy among patients, can hinder effective implementation of eCOA solutions
- Overcoming these challenges through localized deployment strategies, patient training, regulatory alignment, and investment in secure, adaptable platforms is essential for sustained growth of the eCOA market in Asia-Pacific
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) Market Scope
The market is segmented on the basis of product, approach, end user, and platform.
- By Product
On the basis of product, the Asia-Pacific electronic clinical outcome assessment (eCOA) market is segmented into on-premise solutions, cloud-based solutions, and web-based solutions. The cloud-based solutions segment dominated the market with the largest revenue share of 45.6% in 2024, driven by the increasing adoption of digital clinical trial platforms and the flexibility offered for remote and decentralized trials. Cloud-based eCOA platforms allow sponsors, CROs, and hospitals to access patient-reported and clinical outcome data in real time, improving operational efficiency and reducing data entry errors. These platforms also facilitate collaboration among multi-site clinical trials, allowing study managers and investigators to track progress, monitor compliance, and address queries instantly. The market sees strong demand for cloud-based solutions due to their scalability, minimal IT infrastructure requirements, and cost-effectiveness for both large and small-scale trials.
The web-based solutions segment is anticipated to witness the fastest growth rate of 23.4% from 2025 to 2032, fueled by the rising need for browser-accessible platforms that support diverse trial environments. These platforms provide user-friendly interfaces for patients, clinicians, and study coordinators, reducing the learning curve for electronic data capture. Web-based eCOA systems also allow remote access to trial data, which is particularly important for decentralized and hybrid clinical trials, improving patient engagement and adherence. The flexibility of web-based platforms ensures that trials can be conducted in regions with varying levels of technological infrastructure. The adoption of web-based solutions is also encouraged by regulatory agencies emphasizing electronic data capture for patient-reported outcomes.
- By Approach
On the basis of approach, the Asia-Pacific electronic clinical outcome assessment (eCOA) market is segmented into Clinician Reported Outcome Assessment (CLINRO), Patient Reported Outcome Assessment (PRO), Observer Reported Outcome Assessment (OBSRO), and Performance Outcome Assessment (PERFO). The Patient Reported Outcome Assessment (PRO) segment dominated the market with a significant revenue share of 42.8% in 2024, driven by its importance in capturing real-world patient experiences and satisfaction levels. PRO solutions enable participants to report symptoms, side effects, and quality-of-life measures directly, reducing reliance on intermediaries and minimizing reporting bias. This approach is particularly valuable in chronic disease trials and long-term studies where patient feedback is critical for efficacy and safety evaluation. PRO data helps sponsors and regulators make informed decisions while improving patient engagement and adherence. Furthermore, the increasing integration of PRO modules with mobile devices and tablets enhances data collection accuracy and convenience for participants.
The Observer Reported Outcome Assessment (OBSRO) segment is expected to witness the fastest CAGR from 2025 to 2032, as clinical trials increasingly involve pediatric, geriatric, and special populations who cannot reliably self-report outcomes. OBSRO solutions allow caregivers, parents, or trained observers to capture essential clinical and behavioral data on behalf of the patient. This method ensures high-quality, standardized data collection while maintaining patient safety and compliance. The segment benefits from the development of mobile and wearable-assisted OBSRO platforms, enabling real-time data capture and analytics. Increased regulatory emphasis on patient-centric trials and accurate data capture further supports the growth of the OBSRO segment.
- By End User
On the basis of end user, the Asia-Pacific electronic clinical outcome assessment (eCOA) market is segmented into commercial service providers, hospitals and transplant centers, research laboratories, and academic institutions. The commercial service providers segment dominated the market with the largest revenue share of 39.8% in 2024, owing to their pivotal role in conducting outsourced clinical trials for pharmaceutical and biotech companies. These providers leverage eCOA platforms to ensure consistent, accurate, and compliant data capture across multi-site trials, enhancing efficiency and reducing timelines. They also benefit from cloud-based and mobile eCOA solutions that enable centralized monitoring and real-time reporting. Commercial service providers increasingly adopt integrated eCOA solutions to offer comprehensive services, including patient recruitment, engagement, and trial monitoring. Furthermore, their ability to manage large-scale trials and provide robust support infrastructure drives continued investment in advanced eCOA systems.
Hospitals and transplant centers are projected to witness the fastest growth rate of 22.1% from 2025 to 2032 due to rising adoption of eCOA systems for patient monitoring, electronic record-keeping, and integration with hospital information systems. These institutions are increasingly participating in multi-center clinical trials and require standardized platforms for data collection and reporting. eCOA solutions enable clinicians to monitor patients remotely, capture real-time outcomes, and improve compliance. Integration with electronic health records (EHRs) ensures seamless transfer of clinical data while maintaining regulatory compliance. Hospitals also benefit from improved patient engagement through mobile PRO modules, which enhance trial retention and data reliability.
- By Platform
On the basis of platform, the Asia-Pacific electronic clinical outcome assessment (eCOA) market is segmented into Contract Research Organizations (CROs), pharmaceutical and biopharmaceutical companies, medical device manufacturers, hospitals and clinical laboratories, consulting service companies, research and academia, and others. The CRO segment dominated the market with a revenue share of 41.5% in 2024, driven by the growing outsourcing of clinical trials and their reliance on eCOA platforms for patient-centric data collection, regulatory compliance, and operational efficiency. CROs increasingly prefer integrated digital trial solutions that combine eCOA, eConsent, and remote monitoring capabilities. This approach reduces trial timelines, enhances data quality, and allows seamless collaboration with sponsors and sites across multiple regions. In addition, CROs invest in cloud-based and mobile eCOA solutions to support decentralized and hybrid trial models. The need for real-time monitoring, analytics, and reporting further strengthens the adoption of eCOA solutions in CRO operations.
Pharmaceutical and biopharmaceutical companies are expected to register the fastest CAGR from 2025 to 2032, supported by the increasing focus on digital clinical trial solutions and patient-reported outcomes to accelerate drug development timelines. These companies are leveraging eCOA platforms to capture accurate efficacy and safety data, optimize trial design, and improve regulatory submission readiness. Integration with other digital solutions such as clinical trial management systems (CTMS) and electronic data capture (EDC) systems enhances operational efficiency. In addition, pharmaceutical sponsors are increasingly adopting patient-centric trial models, which require robust eCOA systems to collect real-time, high-quality data.
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) Market Regional Analysis
- China dominated the Asia-Pacific electronic clinical outcome assessment (eCOA) market with the largest revenue share of 39% in 2024, characterized by increasing clinical trial activities, rapid digital health adoption, and the presence of key industry players, with significant uptake of eCOA solutions in multicenter and decentralized clinical trials
- Clinical researchers and sponsors in the region highly value the accuracy, real-time data access, and patient-centric capabilities offered by eCOA platforms, which streamline data capture and improve compliance in both hospital-based and decentralized trials
- This widespread adoption is further supported by rising investment in healthcare infrastructure, growing collaboration between pharmaceutical companies and CROs, and an increasing number of decentralized and hybrid clinical trials, establishing eCOA solutions as a preferred tool for clinical research across Asia-Pacific
The China eCOA Market Insight
The China electronic clinical outcome assessment (eCOA) market captured the largest revenue share of 38.5% in 2024 within Asia-Pacific, fueled by rapid growth in clinical trial activities, increasing adoption of digital health technologies, and supportive government initiatives promoting electronic patient-reported outcome collection. Clinical researchers and sponsors are increasingly prioritizing real-time, accurate, and patient-centric data capture to streamline trials. Furthermore, the integration of mobile-based platforms and cloud solutions is enhancing accessibility and operational efficiency across multicenter trials.
Japan eCOA Market Insight
The Japan electronic clinical outcome assessment (eCOA) market is gaining momentum due to advanced healthcare infrastructure, high technology adoption, and a growing focus on patient-centric clinical trials. The market emphasizes secure, accurate, and easy-to-use electronic data capture solutions, particularly in hospital-based and decentralized trials. Integration with mobile devices and analytics tools is driving efficiency and compliance, while the aging population is increasing demand for platforms that simplify reporting for elderly patients.
India eCOA Market Insight
The India electronic clinical outcome assessment (eCOA) market accounted for a significant revenue share in Asia-Pacific in 2024, attributed to the country’s expanding clinical research ecosystem, rising investment in digital health technologies, and rapid adoption of mobile and web-based reporting solutions. India is becoming a hub for multinational and local clinical trials, and the growing availability of cost-effective eCOA platforms, coupled with government initiatives promoting digital health, is fueling market growth.
Australia eCOA Market Insight
The Australia electronic clinical outcome assessment (eCOA) market is expected to grow steadily due to increasing focus on decentralized trials, digital patient engagement, and adoption of cloud-based and web-based eCOA platforms. The country’s robust healthcare infrastructure and emphasis on regulatory compliance encourage the use of secure and efficient electronic outcome assessment tools. Integration with hospital information systems and clinical trial management platforms further supports the market expansion.
South Korea eCOA Market Insight
The South Korea electronic clinical outcome assessment (eCOA) market is witnessing growth driven by technological advancements in clinical trials, strong government support for digital health adoption, and increasing demand for patient-reported outcome collection. Hospitals and research institutions are increasingly leveraging mobile and cloud-based eCOA solutions to enhance patient engagement, improve data quality, and accelerate trial timelines. The focus on regulatory compliance and data security is further strengthening market confidence.
Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) Market Share
The Asia-Pacific electronic clinical outcome assessment (eCOA) industry is primarily led by well-established companies, including:
- IQVIA (U.S.)
- Medidata Solutions, Inc. (U.S.)
- Clario (U.S.)
- ArisGlobal (U.S.)
- Signant Health (U.S.)
- TransPerfect (U.S.)
- Cloudbyz (U.S.)
- Climedo Health GmbH (Germany)
- ClinCapture (U.S.)
- Veeva Systems (U.S.)
- ObvioHealth (U.S.)
- Novotech (Australia)
- Parexel International (MA) Corporation (U.S.)
- Anju Software, Inc. (U.S.)
- Kayentis (France)
- The Diary Pte. Ltd (Singapore)
- Bracket Global LLC (U.S.)
- Dassault Systèmes SE (France)
- CRF Health (U.S.)
- ERT Clinical (U.S.)
What are the Recent Developments in Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) Market?
- In May 2025, Clario announced the completion of its acquisition of the electronic clinical outcome assessment (eCOA) business from WCG. This strategic acquisition enhances Clario's capabilities in providing digital endpoint data solutions, particularly in the neuroscience space, by integrating WCG's expertise and technology. The move is expected to strengthen Clario's market position globally, including in the Asia-Pacific region, by offering more comprehensive solutions to its clients
- In February 2024, an IQVIA blog post highlighted the company's strategies for accelerating eCOA implementation to support the rise of decentralized clinical trials (DCTs). The article emphasized the benefits of its extensive library of pre-built and pre-validated assessments, which helps reduce study startup times from several months to a matter of weeks. This focus on agility and efficiency has become a cornerstone of IQVIA's approach in the Asia-Pacific market, where DCTs are gaining traction
- In August 2023, China's National Medical Products Administration (NMPA) and the Center for Drug Evaluation (CDE) formally released the "Technical Guidelines for the Implementation of Patient-Centered Drug Clinical Trials." These guidelines provide a framework for using digital tools such as eCOA, electronic informed consent, and remote data collection, signaling the Chinese government's official support for modernizing clinical trial practices. This regulatory clarity is expected to drive increased adoption of eCOA technologies in the country
- In April 2023, Almac Clinical Technologies, a member of the Almac Group, announced the launch of its IXRS3 Partnership Network. This initiative aims to expand the company's capabilities in providing advanced electronic Clinical Outcome Assessment (eCOA) solutions across the Asia-Pacific region. The network is designed to accelerate clinical trials by integrating innovative technology and expertise, thereby enhancing the efficiency and effectiveness of eCOA implementations in the region
- In March 2021, Veeva Systems announced that LSK Global Pharma Services Co. Ltd., a leading Korean CRO, had adopted Veeva Vault eTMF, Veeva Vault CTMS, and Veeva SiteVault Free. This move was a part of Veeva's broader strategy to expand its integrated cloud-based clinical solutions in the Asia-Pacific region, helping local companies streamline trial management and collaborate more effectively on a global scale
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.