Asia Pacific Single Use Medical Devices Reprocessing Market
Market Size in USD Billion
CAGR :
% 
 USD
1.14 Billion
USD
4.19 Billion
2025
2033
USD
1.14 Billion
USD
4.19 Billion
2025
2033
| 2026 –2033 | |
| USD 1.14 Billion | |
| USD 4.19 Billion | |
|
|
|
|
Asia-Pacific Single Use Medical Devices Reprocessing Market Size
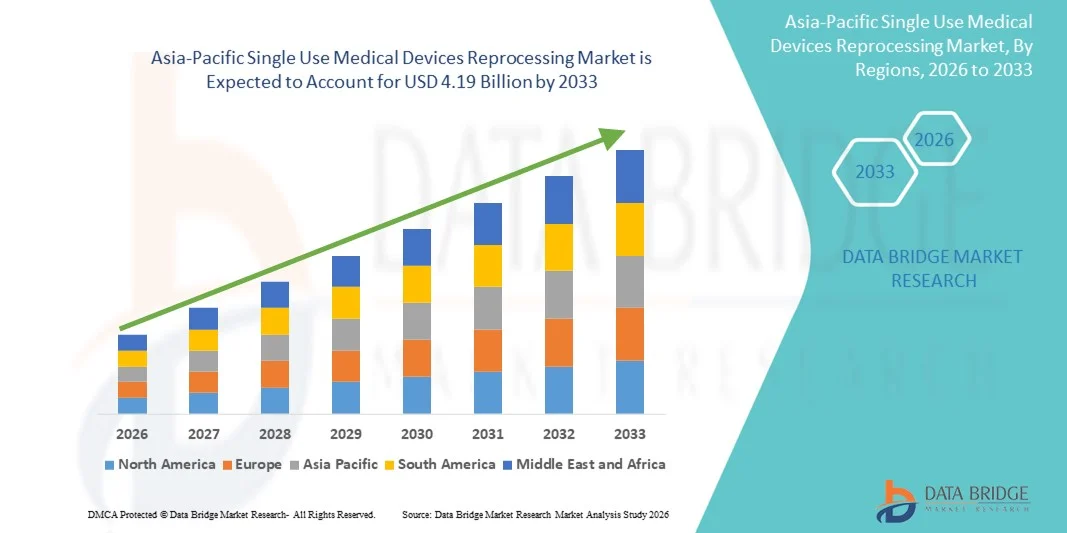
- The Asia-Pacific single use medical devices reprocessing market size was valued at USD 1.14 billion in 2025 and is expected to reach USD 4.19 billion by 2033, at a CAGR of 17.70% during the forecast period
- The market growth is largely driven by the increasing adoption of cost-effective and sustainable healthcare practices, coupled with technological advancements in medical device sterilization and reprocessing, leading to enhanced operational efficiency and patient safety in hospitals, clinics, and diagnostic centers
- Furthermore, rising demand for reducing medical waste, lowering healthcare costs, and ensuring compliance with regulatory standards for device safety is accelerating the uptake of Single Use Medical Devices Reprocessing solutions, thereby significantly boosting the overall growth of the Single Use Medical Devices Reprocessing Market
Asia-Pacific Single Use Medical Devices Reprocessing Market Analysis
- Single Use Medical Devices Reprocessing solutions, which enable safe, cost-effective, and compliant reprocessing of disposable medical devices, are increasingly critical in modern healthcare systems due to rising medical costs, regulatory pressures, and the need to reduce medical waste
- The market growth is primarily driven by advancements in sterilization and reprocessing technologies, increasing adoption of sustainable healthcare practices, and growing awareness among hospitals and clinics about patient safety and regulatory compliance
- China dominated the single use medical devices reprocessing market with a revenue share of approximately 37.8% in 2025, supported by a large healthcare infrastructure, high patient volumes, government initiatives for hospital modernization, and strong adoption of advanced reprocessing technologies
- India is expected to be the fastest-growing region during the forecast period, owing to expanding hospital networks, rising healthcare expenditure, increasing awareness of infection control, and growing adoption of reprocessing solutions in urban and semi-urban areas
- The In-House segment dominated the largest market revenue share of 55.1% in 2025, as hospitals prefer direct oversight of reprocessing operations to ensure quality, compliance, and faster turnaround
Report Scope and Single Use Medical Devices Reprocessing Market Segmentation
|
Attributes |
Single Use Medical Devices Reprocessing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
• Stryker Corporation (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Asia-Pacific Single Use Medical Devices Reprocessing Market Trends
Technological Advancements and Focus on Safety in Single Use Medical Devices Reprocessing
- A significant and accelerating trend in the Asia-Pacific Single Use Medical Devices Reprocessing market is the adoption of advanced sterilization, cleaning, and validation technologies to ensure safe and effective reuse of single-use medical devices
- For instance, in June 2024, Stryker India implemented an automated reprocessing system in its multi-specialty hospitals, reducing human error and improving sterilization efficiency across surgical departments
- There is a growing emphasis on eco-friendly and cost-effective reprocessing methods, including low-temperature sterilization techniques and disposable device tracking systems, which minimize environmental impact while enhancing patient safety
- Standardization initiatives and regulatory compliance frameworks are shaping the development of reprocessing protocols, ensuring adherence to international standards such as ISO 13485 and FDA guidelines
- The trend toward more sophisticated, efficient, and safe reprocessing solutions is driving adoption in hospitals, diagnostic centers, and surgical facilities, enabling healthcare providers to reduce medical waste and operational costs
Asia-Pacific Single Use Medical Devices Reprocessing Market Dynamics
Driver
Rising Demand for Cost Reduction and Efficient Healthcare Operations
- The increasing healthcare expenditure in Asia-Pacific, coupled with the need to optimize operational efficiency in hospitals, is a major driver for the Single Use Medical Devices Reprocessing market
- For instance, in March 2025, Fortis Healthcare, India, upgraded its surgical units with advanced reprocessing equipment, achieving a 20% reduction in device procurement costs while ensuring compliance with sterilization standards
- Healthcare providers are under pressure to manage rising medical costs while maintaining high standards of patient care, making reprocessing of single-use devices an attractive solution
- Furthermore, the growing number of surgical procedures and diagnostic interventions in the region is driving demand for reliable, safe, and fast reprocessing solutions to meet operational requirements
- Increasing awareness of environmental sustainability is also motivating healthcare facilities to adopt reprocessing technologies that reduce medical waste and improve resource utilization
Restraint/Challenge
Strict Regulatory Compliance and Risk of Contamination
- Stringent regulatory requirements across different countries in the Asia-Pacific region, such as FDA, TGA, and national health authorities, pose a challenge for widespread adoption of single-use medical device reprocessing
- For instance, in August 2024, a private hospital network in Malaysia postponed implementing a new reprocessing system due to delays in obtaining certification from the Ministry of Health and completing mandatory compliance audits
- The risk of contamination or infection due to improper reprocessing remains a critical concern, which can impact patient safety and limit adoption
- High capital expenditure for advanced reprocessing equipment and ongoing maintenance costs can be barriers for smaller healthcare providers or clinics in developing regions
- Limited trained personnel capable of performing standardized reprocessing protocols may further slow market growth
- Overcoming these challenges through robust training programs, cost-effective solutions, and clear regulatory guidance will be essential for sustained growth of the Single Use Medical Devices Reprocessing market
Asia-Pacific Single Use Medical Devices Reprocessing Market Scope
The market is segmented on the basis of product type, price range, application, type, end-user, and distribution channel.
- By Product Type
On the basis of product type, the Asia-Pacific Single Use Medical Devices Reprocessing market is segmented into Class I Devices and Class II Devices. The Class I Devices segment dominated the largest market revenue share of 46.5% in 2025, owing to the high-volume utilization of devices such as syringes, catheters, gloves, and cannulas in daily medical procedures. These devices are simpler in design, easier to sterilize, and involve lower risk during reprocessing, which has led to widespread adoption across hospitals and ambulatory surgical centers in China, India, Japan, and Australia. Regulatory frameworks in these countries are increasingly supporting safe reprocessing practices, further boosting demand. Hospitals also prefer Class I device reprocessing to reduce operational costs and manage waste effectively. Technological advances in sterilization methods, quality control protocols, and traceability systems have enhanced safety, thereby building confidence among end-users. Additionally, Class I reprocessing enables healthcare providers to sustainably manage device inventories while ensuring compliance with international safety standards.
The Class II Devices segment is expected to witness the fastest CAGR of 22.3% from 2026 to 2033, driven by the growing usage of high-risk devices such as endoscopes, ventilators, surgical staplers, and implantable instruments that require stringent sterilization and validation processes. Hospitals and surgical centers increasingly outsource Class II device reprocessing to certified third-party providers to ensure compliance with safety regulations and reduce operational burdens. The expansion of specialty clinics and private hospitals in India, China, and Southeast Asia contributes to growth, alongside improvements in reprocessing technology, automated sterilization systems, and real-time monitoring solutions. Rising awareness regarding patient safety and cost reduction in healthcare supply chains further fuels adoption of Class II device reprocessing, supporting long-term market growth in the Asia-Pacific region.
- By Price Range
On the basis of price range, the market is segmented into High Range and Low/Economy Range. The High Range segment dominated the largest market revenue share of 51.2% in 2025, driven by the reprocessing of advanced devices like cardiovascular catheters, arthroscopy tools, and anesthesia instruments that demand meticulous handling, strict sterilization protocols, and compliance with regulatory standards. Hospitals in urban and semi-urban areas of Japan, China, South Korea, and Australia prefer high-range device reprocessing for premium-quality instruments, as it ensures longevity, reduces costs of frequent replacements, and minimizes medical waste. The high adoption is also due to robust investments in healthcare infrastructure, growing awareness of infection control, and rising patient expectations for safe and reliable medical procedures. Strategic partnerships between hospitals and reprocessing service providers further consolidate the dominance of high-range devices in market revenue.
The Low/Economy Range segment is expected to witness the fastest CAGR of 19.8% from 2026 to 2033, fueled by increasing demand from rural hospitals, smaller clinics, and cost-sensitive healthcare providers across India, Indonesia, and Vietnam. Economy-range devices such as basic catheters, syringes, and gloves are increasingly reprocessed to reduce operational costs while maintaining compliance with local safety regulations. Rising awareness about sustainable healthcare practices, combined with the growth of outpatient care centers and smaller surgical units, encourages adoption of budget-friendly reprocessing solutions. Technological advancements in sterilization and process validation have also made it safer and more efficient to reprocess economy-range devices, contributing to rapid market expansion.
- By Application
On the basis of application, the market is segmented into General Surgery, Anesthesia, Arthroscopy & Orthopaedic Surgery, Cardiology, Gastroenterology, Urology, Gynecology, and Others. The General Surgery segment dominated the largest market revenue share of 48.9% in 2025, due to the routine, high-volume use of single-use devices in surgeries, including scalpels, surgical gloves, sutures, and basic catheters. Hospitals and surgical centers in Japan, China, India, and Australia prioritize reprocessing for general surgery instruments to minimize costs while maintaining quality and regulatory compliance. The adoption is also driven by increasing surgical procedures, rising patient volumes, and healthcare expansion in urban areas. Technological advancements in sterilization and automated reprocessing equipment enhance efficiency, accuracy, and safety of General Surgery device reprocessing, further supporting revenue dominance.
The Arthroscopy & Orthopaedic Surgery segment is expected to witness the fastest CAGR of 21.5% from 2026 to 2033, driven by increasing prevalence of musculoskeletal disorders, sports-related injuries, and aging populations in Asia-Pacific. Devices like arthroscopes, joint replacement instruments, and orthopedic tools require meticulous reprocessing to maintain functional integrity and patient safety. Hospitals and specialty clinics increasingly outsource complex reprocessing, leveraging advanced sterilization techniques and automated workflows. Expansion of private healthcare networks in India, Southeast Asia, and South Korea, along with rising government initiatives promoting sustainable healthcare, further fuels adoption of reprocessed devices in these surgical applications, ensuring rapid growth in this segment.
- By Type
On the basis of type, the market is segmented into In-House and Outsource. The In-House segment dominated the largest market revenue share of 55.1% in 2025, as hospitals prefer direct oversight of reprocessing operations to ensure quality, compliance, and faster turnaround. Large hospitals and multi-specialty centers in China, Japan, and Australia benefit from in-house reprocessing due to better control over sterilization, monitoring, and documentation processes. Adoption is also driven by cost-effectiveness in high-volume hospitals, stringent regulatory requirements, and the need for rapid device availability. In-house reprocessing allows hospitals to implement standardized protocols, reduce errors, and enhance patient safety, consolidating the segment’s dominance.
The Outsource segment is expected to witness the fastest CAGR of 23.0% from 2026 to 2033, fueled by increasing reliance of mid-sized hospitals and ambulatory surgical centers on third-party providers. Outsourced reprocessing offers certified, scalable, and cost-effective solutions while ensuring compliance with strict regulatory guidelines. Countries such as India, South Korea, and Southeast Asia have witnessed rapid growth in outsourced reprocessing due to infrastructure limitations and rising patient volumes. Technological integration, automation in sterilization, and traceability systems have made outsourcing safer and more reliable, further driving growth in this segment.
- By End User
On the basis of end-user, the market is segmented into Hospitals, Ambulatory Surgical Centers, and Others. Hospitals accounted for the largest market revenue share of 60.3% in 2025, due to high device volumes, routine procedures, and strict adherence to reprocessing regulations. Adoption is high in urban hospitals across China, Japan, and Australia, where cost reduction, operational efficiency, and patient safety are top priorities. Hospitals implement structured reprocessing protocols to handle Class I and Class II devices, ensuring compliance and maximizing device life cycles.
The Ambulatory Surgical Centers segment is expected to witness the fastest CAGR of 22.7% from 2026 to 2033, driven by the growing number of outpatient procedures and clinics outsourcing reprocessing to minimize capital investment and ensure compliance. Private and semi-private centers in India, South Korea, and Southeast Asia increasingly adopt third-party reprocessing services, supported by cost-efficiency, technological safety, and regulatory alignment. Rising outpatient healthcare demand and government encouragement for sustainable practices further accelerate this segment’s growth.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into B2B and B2C. The B2B segment dominated the largest market revenue share of 66.0% in 2025, as hospitals and surgical centers primarily procure bulk reprocessing services from certified providers to ensure quality, compliance, and operational efficiency. Large hospital chains in China, Japan, and Australia prefer contractual agreements with service providers, ensuring consistent device availability and standardized procedures. The B2B model allows for bulk pricing, streamlined logistics, and predictable supply, which is crucial for high-volume facilities. Hospitals and multispecialty centers benefit from dedicated account management, training, and technical support offered by providers, enhancing process reliability. Additionally, B2B agreements often include customized sterilization protocols for Class I and Class II devices, ensuring regulatory compliance. The model supports long-term partnerships, fostering trust between hospitals and reprocessing providers. Adoption is further driven by the need to minimize operational downtime, maintain device traceability, and comply with stringent local and international regulations. Technological integration such as automated tracking, digital audits, and IoT-enabled sterilization systems further strengthens the dominance of the B2B distribution channel in the Asia-Pacific region.
The B2C segment is expected to witness the fastest CAGR of 20.5% from 2026 to 2033, driven by smaller clinics and individual practitioners increasingly engaging certified reprocessing providers. Growth is supported by rising awareness about sustainable healthcare practices, government incentives, and easy access to third-party reprocessing solutions in India, Southeast Asia, and South Korea. Smaller facilities, including specialty clinics and outpatient centers, find B2C services convenient for handling limited device volumes without investing in in-house reprocessing infrastructure. The model allows these providers to access certified sterilization services on-demand, reducing capital expenditure and operational risk. Increasing adoption of e-commerce and digital platforms in healthcare supplies facilitates faster ordering, tracking, and delivery of reprocessed devices. The B2C channel also benefits from flexible pricing models, subscription services, and educational support from providers, ensuring compliance and quality assurance. As patient safety and infection control remain high priorities, B2C reprocessing services are gaining credibility and trust, driving rapid adoption across emerging markets in the Asia-Pacific region.
Asia-Pacific Single Use Medical Devices Reprocessing Market Regional Analysis
- The Asia-Pacific single use medical devices reprocessing market is projected to register robust growth during the forecast period
- Driven by rising healthcare demand, expanding hospital and diagnostic infrastructure, increasing adoption of advanced reprocessing technologies, and growing awareness regarding infection prevention
- The market growth is further supported by improving healthcare expenditure and modernization initiatives across emerging economies such as China and India
China Single Use Medical Devices Reprocessing Market Insight
China single use medical devices reprocessing market dominated the Single Use Medical Devices Reprocessing market with a revenue share of approximately 37.8% in 2025, supported by a large healthcare infrastructure, high patient volumes, government initiatives for hospital modernization, and strong adoption of advanced reprocessing technologies. Hospitals and diagnostic centers in the region highly value the efficiency, cost-effectiveness, and safety provided by single use medical device reprocessing systems, enabling better infection control and sustainable use of medical equipment. This widespread adoption is further supported by continuous investments in healthcare infrastructure, rising awareness about infection prevention, and government-led initiatives to modernize hospitals, establishing reprocessing solutions as essential in clinical and diagnostic settings.
India Single Use Medical Devices Reprocessing Market Insight
The India single use medical devices reprocessing market is expected to be the fastest-growing region during the forecast period, owing to expanding hospital networks, rising healthcare expenditure, increasing awareness of infection control, and growing adoption of reprocessing solutions in urban and semi-urban areas. The modernization of hospitals and diagnostic centers, along with government support for infection prevention programs, is accelerating market growth across the country.
Asia-Pacific Single Use Medical Devices Reprocessing Market Share
The Single Use Medical Devices Reprocessing industry is primarily led by well-established companies, including:
• Stryker Corporation (U.S.)
• Getinge AB (Sweden)
• Advanced Sterilization Products (U.S.)
• 3M Company (U.S.)
• Tuttnauer Europe (Israel)
• Matachana Group (Spain)
• Sterigenics International (U.S.)
• ASP, Inc. (U.S.)
• MMM Group (Germany)
• Johnson & Johnson (U.S.)
• Becton, Dickinson and Company (U.S.)
• Olympus Corporation (Japan)
• Wassenburg Medical (Netherlands)
• Medivators (U.S.)
• Getinge USA (U.S.)
• STERIS Corporation (U.S.)
• Sterisafe Solutions (India)
• Hirayama Instruments (Japan)
Latest Developments in Asia-Pacific Single Use Medical Devices Reprocessing Market
- In February 2023, Northeast Scientific Inc. received FDA 510(k) clearance for reprocessing the Philips IVUS Eagle Eye Platinum RX Digital Catheter, enabling hospitals and catheterization laboratories across the United States to safely reprocess and reuse a previously single‑use intravascular ultrasound device, leading to substantial cost savings and improved operational efficiency. This clear regulatory milestone helped expand the scope of reprocessed cardiovascular devices, reinforcing industry confidence in safety‑validated reuse under FDA oversight
- In March 2023, SterilMed launched a new line of electrophysiology catheters equipped with integrated RFID tracking, allowing healthcare providers to precisely monitor device lifecycle, usage history, and sterilization status across over 120 facilities in North America, improving inventory management and compliance. The RFID integration reflects broader adoption of smart tracking technologies in reprocessing workflows to enhance traceability and patient safety
- In October 2023, Vanguard AG opened a fully automated sterilization hub in Germany, increasing reprocessed device throughput by approximately 35% while reducing manual labor requirements by 28%, demonstrating strong investment in automation to scale reprocessing operations and support sustainability goals. The Germany facility also aligns with EU regulations aimed at reducing medical waste and reinforcing controlled reprocessing practices
- In February 2024, Medline ReNewal introduced an AI‑powered sterilization validation software adopted by over 45 hospitals in the United States, cutting sterilization errors by 22% and strengthening compliance with regulatory standards while advancing real‑time quality verification across reprocessing cycles. This development underscores the role of artificial intelligence in validating complex reprocessing workflows and improving device safety metrics
- In July 2024, ReNu Medical implemented cloud‑based reprocessing monitoring across 60 outpatient surgical centers in Europe, achieving a roughly 25% reduction in inventory losses and improving real‑time device traceability, marking a significant shift toward digital supply chain integration in reprocessing programs. Cloud adoption is enhancing data transparency and operational coordination between clinics and third‑party providers
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.