Europe Aesthetic Energy Based Device Market
Market Size in USD Million
CAGR :
% 
 USD
65.51 Million
USD
614.87 Million
2022
2030
USD
65.51 Million
USD
614.87 Million
2022
2030
| 2023 –2030 | |
| USD 65.51 Million | |
| USD 614.87 Million | |
|
|
|
|
Europe Aesthetic Energy-Based Device Market Analysis and Size
Rising geriatric adoption, rising public awareness about cosmetic procedures, the availability of technologically advanced and user-friendly products, and rising demand for aesthetic treatments among men are expected to drive the growth of the aesthetic services market between 2023 and 2030. On the other hand, rising adoption and availability of unusual beauty and cosmetic products open up new avenues for growth in the aesthetic services market during the forecast period.
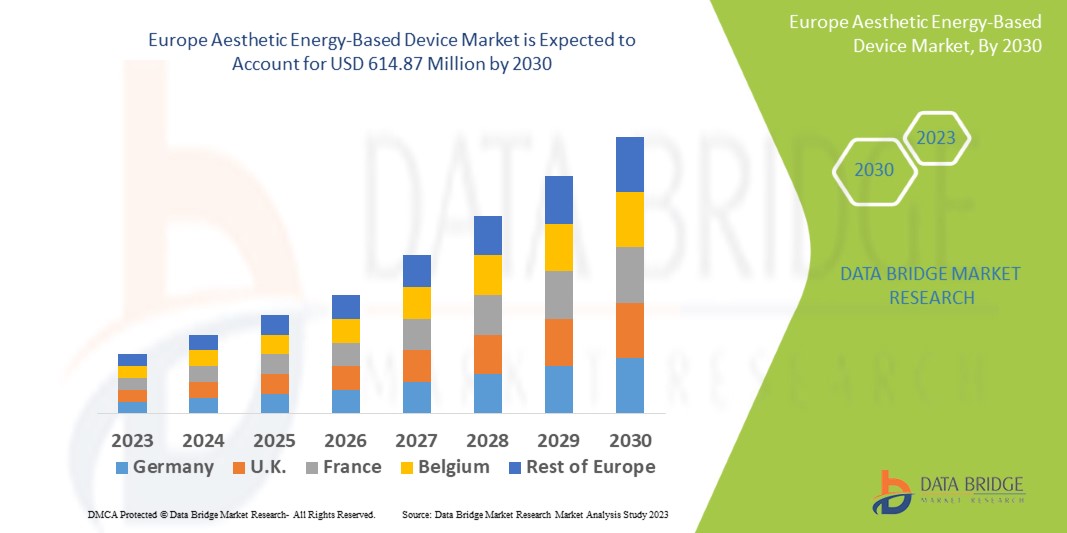
Data Bridge Market Research analyses that the aesthetic energy-based device market which is USD 65.51 million in 2022, is expected to reach USD 614.87 million by 2030, at a CAGR of 32.3% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Europe Aesthetic Energy-Based Device Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product (Devices and Consumables), Technology (Laser-Based Technology, Light-Laser Based Technology, Dynamic Pulse Control Technology, Intense Pulse Light Technology, Energy-Based Technology, UV Technology, Infrared Technology, Radiofrequency Technology, Low Temperature-Based Technology, Cryolipolysis, Suction-Based, Plasma Energy-Based Devices and Others), Procedure (Cosmetic Procedures and Reconstructive Procedures), Application (Surgical and Non-Surgical), End User (Hospital, Specialty Clinics, Medical Spas and Beauty Centers, Dermatology Clinics, Academic and Research Institute and Others), Distribution Channel (Direct Tender and Retail Sales) |
|
Countries Covered |
Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe |
|
Market Players Covered |
FACE Aesthetic Artistry (U.S.), Mayo Foundation for Medical Education and Research (MFMER) (India), The Ottawa Skin Clinic (Canada), VIVA Skin Clinics (U.K.), Mirror Mirror Beauty Boutique (U.S.), International Association of Better Business Bureaus, Inc. (U.S.), Saltz Plastic Surgery (India), Mark L. Jewell, MD (U.S.), Crystal Clear Digital Marketing (U.S.), Azul Cosmetic Surgery and Medical Spa (U.S.) |
|
Market Opportunities |
|
Market Definition
Medical aesthetic devices include all medical devices used for cosmetic procedures such as plastic surgery, unwanted hair removal, excess fat removal, anti-aging, aesthetic implants, skin tightening, and others that are used for body beautification, correction, and improvement. Aesthetic procedures can be both surgical and non-surgical. Liposuction, breast implants, facelifts, radiofrequency, and other procedures are included in the surgical procedure. Chemical peels, non-surgical liposuction, and skin tightening procedures are instances of non-surgical procedures.
Europe Aesthetic Energy-Based Device Market Dynamics
- Rise in geriatric population
Wrinkles, loss of skin suppleness, and dark spots appear between the ages of 25 and 30, and between the ages of 30 and 65, wrinkles, loss of skin suppleness, and dark patches become more visible. As a result, a large population susceptible to various signs of ageing drives up demand for aesthetic medicines. According to the CIA World Factbook, approximately 30.5 percent of the total German population was aged 25 to 54 in 2018, with approximately 13.6 percent of the population aged 55 to 65. As a result, the market for aesthetic energy-based devices among adults aged 25 to 65 is growing steadily.
- Increasing demand for minimally invasive procedures
Rising demand for body feature enhancement and increased adoption of new technologies drive market expansion. Another major factor driving industry growth is people's growing self-consciousness about their appearance. A more sedentary lifestyle, particularly in terms of nutrition and physical activity, is becoming a major contributor to the rise in obesity and weight-related disorders. This contributes to market expansion.
Opportunities
- Rising demand for aesthetic procedures
Rising disposable income, increased social awareness, increased demand for these procedures in the entertainment industry, and increased traumatic injuries requiring reconstructive changes are all factors driving the market. For instance, Allergen Aesthetics received FDA approval in June 2021 for Juvederm Voluma for China region augmentation in adults over the age of 18.
Restraints/Challenges
- Risks associated with aesthetic procedures
Complications from medical aesthetic procedures and clinical risks are likely to stymie the growth of the aesthetic energy-based device market during the forecast period. The ethical and social issues associated with cosmetic treatments pose the most significant challenge to the aesthetic energy-based device market.
This aesthetic energy-based device market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the aesthetic energy-based device market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In 2019, Lumenis Ltd. launched Legend Pro+, a multi-application programme with cutting-edge technology. It is a platform for the body and face that offers comfortable solutions, immediate results, minimal pain and downtime, and long-term efficacy. Furthermore, the product can be used without anaesthesia.
Europe Aesthetic Energy-Based Device Market Scope
The aesthetic energy-based device market is segmented on the basis of product, technology, procedure, application, end user and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Devices
- Consumables
Technology
- Laser-Based Technology
- Light-Laser Based Technology
- Dynamic Pulse Control Technology
- Intense Pulse Light Technology
- Energy-Based Technology
- UV Technology
- Infrared Technology
- Radiofrequency Technology
- Low Temperature-Based Technology
- Cryolipolysis
- Suction-Based
- Plasma Energy-Based Devices
- Others
Procedure
- Cosmetic Procedures
- Reconstructive Procedures
Application
- Surgical
- Non-Surgical
End User
- Hospital
- Specialty Clinics
- Medical Spas and Beauty Centers
- Dermatology Clinics
- Academic and Research Institute
- Others
Distribution Channel
- Direct Tender
- Retail Sales
Europe Aesthetic Energy-Based Device Market Regional Analysis/Insights
The aesthetic energy-based device market is analyzed and market size insights and trends are provided by country, product, technology, procedure, application, end user and distribution channel as referenced above.
The countries covered in the aesthetic energy-based device market report are Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe.
Germany has the largest market share in Europe's aesthetic energy-based device market for the devices segment, as aesthetic energy-based devices are used for a variety of cosmetic procedures such as plastic surgery, unwanted hair removal, excess fat removal, anti-aging, aesthetic implants, skin tightening, and others that are used for body beautification, correction, and improvement.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The aesthetic energy-based device market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for aesthetic energy-based device market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the aesthetic energy-based device market. The data is available for historic period 2011-2021.
Competitive Landscape and Aesthetic Energy-Based Device Market Share Analysis
The aesthetic energy-based device market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to aesthetic energy-based device market.
Some of the major players operating in the aesthetic energy-based device market are:
- FACE Aesthetic Artistry (U.S.)
- Mayo Foundation for Medical Education and Research (MFMER) (India)
- The Ottawa Skin Clinic (Canada)
- VIVA Skin Clinics (U.K.)
- Mirror Mirror Beauty Boutique (U.S.)
- International Association of Better Business Bureaus, Inc. (U.S.)
- Saltz Plastic Surgery (India)
- Mark L. Jewell, MD (U.S.)
- Crystal Clear Digital Marketing (U.S.)
- Azul Cosmetic Surgery and Medical Spa (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES DATA VOLUME
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER'S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNOLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 FUTURE OUTLOOK
11 REGULATORY COMPLIANCE
11.1 REGULATORY AUTHORITIES
11.2 REGULATORY CLASSIFICATIONS
11.2.1 CLASS I
11.2.2 CLASS II
11.2.3 CLASS III
11.3 REGULATORY SUBMISSIONS
11.4 INTERNATIONAL HARMONIZATION
11.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
11.6 REGULATORY CHALLENGES AND STRATEGIES
12 REIMBURSEMENT FRAMEWORK
13 OPPUTUNITY MAP ANALYSIS
14 INSTALLED BASE DATA
15 VALUE CHAIN ANALYSIS
16 HEALTHCARE ECONOMY
16.1 HEALTHCARE EXPENDITURE
16.2 CAPITAL EXPENDITURE
16.3 CAPEX TRENDS
16.4 CAPEX ALLOCATION
16.5 FUNDING SOURCES
16.6 INDUSTRY BENCHMARKS
16.7 GDP RATION IN OVERALL GDP
16.8 HEALTHCARE SYSTEM STRUCTURE
16.9 GOVERNMENT POLICIES
16.1 ECONOMIC DEVELOPMENT
17 EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET, BY PRODUCT TYPE
17.1 OVERVIEW
17.2 LASER-BASED
17.2.1 BY LASER TYPE
17.2.1.1. DIODE LASER
17.2.1.2. FRACTIONAL CO2 LASER
17.2.1.3. ND:YAG (NEODYMIUM-DOPED YTTRIUM ALUMINIUM GARNET)
17.2.1.4. ERBIUM YAG (ER:YAG)
17.2.1.5. Q-SWITCHED RUBY LASER (QSRL)
17.2.1.6. POTASSIUM TITANYL PHOSPHATE
17.2.1.7. ALEXANDRITE
17.2.1.8. OTHERS
17.2.2 BY MODALITY
17.2.2.1. FIXED
17.2.2.1.1. TABLE TOP
17.2.2.1.2. FLOOR STANDING
17.2.2.2. MOBILE
17.2.2.2.1. TROLLY MOUNTED
17.2.2.2.2. HAND HELD
17.2.3 BY TECHNOLOGY
17.2.3.1. ABLATIVE LASER
17.2.3.2. NON-ABLATIVE LASER
17.2.4 BY PULSE DURATION
17.2.4.1. MILLISECOND
17.2.4.2. PICOSECOND
17.2.4.3. NANOSECOND
17.2.4.4. OTHERS
17.2.5 BY CLASS
17.2.5.1. CLASS I
17.2.5.2. CLASS 2
17.2.5.3. CLASS IIIB
17.2.5.4. CLASS IV
17.2.6 OTHERS
17.3 RADIOFREQUENCY-BASED DEVICES
17.3.1 BY TYPE
17.3.1.1. MONOPOLAR RADIOFREQUENCY
17.3.1.1.1. BY TIP TYPE
17.3.1.1.1.1 V TIP
17.3.1.1.1.2 I TIP
17.3.1.1.1.3 S TIP
17.3.1.1.1.4 F TIP
17.3.1.1.2. BY MODALITY
17.3.1.1.2.1 PORTABLE DEVICES
17.3.1.1.2.2 NON-PORTABLE DEVICES
17.3.1.1.3. OTHERS
17.3.1.2. BIPOLAR RADIOFREQUENCY
17.3.1.2.1. BY TYPE
17.3.1.2.1.1 WITH BROADBAND LIGHT
17.3.1.2.1.2 WITH DIODE LASER
17.3.1.2.2. BY MODALITY
17.3.1.2.2.1 PORTABLE DEVICES
17.3.1.2.2.2 NON-PORTABLE DEVICES
17.3.1.2.3. OTHERS
17.3.1.3. MULTIPOLAR-RADIOFREQUENCY WITH PULSED ELECTROMAGNETIC FIELD
17.3.1.3.1. BY TYPE
17.3.1.3.1.1 WIRED DEVICES
17.3.1.3.1.2 WIRELESS DEVICES
17.3.1.3.2. BY MODALITY
17.3.1.3.2.1 PORTABLE DEVICES
17.3.1.3.2.2 NON-PORTABLE DEVICES
17.3.1.3.3. BY ELECTRODES
17.3.1.3.3.1 THREE ELECTRODES
17.3.1.3.3.2 MORE THAN THREE ELECTRODES
17.3.1.3.4. OTHERS
17.3.2 BY TECHNOLOGY
17.3.2.1. STANDARD RADIOFREQUENCY MICRONEEDLING
17.3.2.2. AI IN RADIOFREQUENCY MICRONEEDLING
17.3.3 OTHERS
17.4 ULTRASOUND BASED DEVICES
17.4.1 BY TYPE
17.4.1.1. STANDARD ULTRASOUND BASED DEVICES
17.4.1.2. HIGH-INTENSITY FOCUSED ULTRASOUND BASED DEVICES
17.4.2 BY MODALITY
17.4.2.1. FIXED
17.4.2.1.1. TABLE TOP
17.4.2.1.2. FLOOR STANDING
17.4.2.2. MOBILE
17.4.2.2.1. TROLLY MOUNTED
17.4.2.2.2. HAND HELD
17.4.3 BY CONNECTIVITY
17.4.3.1. WIRED DEVICES
17.4.3.2. WIRELESS DEVICES
17.4.4 BY FREQUENCY
17.4.4.1. HIGH FREQUENCY
17.4.4.2. ULTRA HIGH FREQUENCY
17.4.5 BY BATTERY
17.4.5.1. INTEGRATED BATTERY
17.4.5.2. DETACHABLE BATTERY
17.4.6 OTHERS
17.5 LIGHT-BASED DEVICES
17.5.1 BY TYPE
17.5.1.1. AESTHETIC MEDICINE LAMP
17.5.1.2. AESTHETIC PHOTOTHERAPY LAMP
17.5.2 BY TECHNOLOGY
17.5.2.1. DYNAMIC PULSE CONTROL (DPC) TECHNOLOGY
17.5.2.2. INTENSE PULSED LIGHT (IPL) TECHNOLOGY
17.5.2.3. PLASMA ENERGY-BASED DEVICES
17.5.3 BY MODALITY
17.5.3.1. FIXED
17.5.3.1.1. TABLE TOP
17.5.3.1.2. FLOOR STANDING
17.5.3.2. MOBILE
17.5.3.2.1. TROLLY MOUNTED
17.5.3.2.2. HAND HELD
17.5.4 BY LIGHT SOURCE
17.5.4.1. LED
17.5.4.2. FLUROSCENT
17.5.4.3. ULTRAVOILET
17.5.5 OTHERS
17.6 CRYOLIPOLYSIS DEVICES
17.6.1 BY MODALITY
17.6.1.1. FIXED
17.6.1.1.1. TABLE TOP
17.6.1.1.2. FLOOR STANDING
17.6.1.2. MOBILE
17.6.1.2.1. TROLLY MOUNTED
17.6.1.2.2. HAND HELD
17.6.2 BY APPLICATORS
17.6.2.1. 6 APPLICATORS
17.6.2.2. 9 APPLICATORS
17.6.2.3. OTHERS
17.6.3 BY APPLICATION
17.6.3.1. BODY SLIMMING
17.6.3.2. BODY SHAPING
17.6.3.3. REMOVE CELLULITE
17.6.3.4. OTHERS
17.6.4 OTHERS
17.7 SUCTION-BASED DEVICES
17.7.1 LIPOSUCTION SURGICAL PUMPS
17.7.1.1. BY MODALITY
17.7.1.1.1. FIXED
17.7.1.1.2. PORTABLE
17.7.1.2. BY FLOW
17.7.1.2.1. 50 I/ MIN
17.7.1.2.2. 55 I/MIN
17.7.1.2.3. 60 I/ MIN
17.7.1.2.4. OTHERS
17.7.1.3. BY JAR CAPACITY
17.7.1.3.1. 4000 ML
17.7.1.3.2. 8000 ML
17.7.1.3.3. OTHERS
17.8 OTHERS AESTHETIC ENERGY BASED DEVICES
18 EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET, BY APPLICATION
18.1 OVERVIEW
18.2 SKIN RESURFACING AND TIGHTENING
18.2.1 BY PRODUCT MODALITY
18.2.1.1. FIXED
18.2.1.2. MOBILE
18.3 BODY CONTOURING AND CELLULITE REDUCTION
18.3.1 BY PRODUCT MODALITY
18.3.1.1. FIXED
18.3.1.2. MOBILE
18.4 HAIR REMOVAL
18.4.1 BY PRODUCT MODALITY
18.4.1.1. FIXED
18.4.1.2. MOBILE
18.5 FACIAL AESTHETIC PROCEDURES
18.5.1 BY PRODUCT MODALITY
18.5.1.1. FIXED
18.5.1.2. MOBILE
18.6 BREAST AUGMENTATION
18.6.1 BY PRODUCT MODALITY
18.6.1.1. FIXED
18.6.1.2. MOBILE
18.7 BREAST RECONSTRUCTION
18.7.1 BY PRODUCT MODALITY
18.7.1.1. FIXED
18.7.1.2. MOBILE
18.8 EYELASHES TREATMENT
18.8.1 BY PRODUCT MODALITY
18.8.1.1. FIXED
18.8.1.2. MOBILE
18.9 PHOTOREJUVENATION
18.9.1 BY PRODUCT MODALITY
18.9.1.1. FIXED
18.9.1.2. MOBILE
18.1 FAT REDUCTION AND BODY CONTOURING
18.10.1 BY PRODUCT MODALITY
18.10.1.1. FIXED
18.10.1.2. MOBILE
18.11 CELLULITE REDUCTION
18.11.1 BY PRODUCT MODALITY
18.11.1.1. FIXED
18.11.1.2. MOBILE
18.12 OTHER APPLICATIONS
19 EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET, BY PROCEDURE
19.1 OVERVIEW
19.2 INVASIVE PROCEDURES
19.3 NON-INVASIVE PROCEDURES
20 EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET, BY MODALITY
20.1 OVERVIEW
20.2 FIXED
20.3 MOBILE
21 EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITALS
21.2.1 PUBLIC
21.2.2 PRIVATE
21.3 SPECIALITY CLINICS
21.4 MEDICAL SPAS & BEAUTY CENTERS
21.5 ACADEMIC AND RESEARCH INSTITUTES
21.6 DERMATOLOGY CLINICS
21.7 OTHERS
22 EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET, BY DISTRIBUTION CHANNEL
22.1 OVERVIEW
22.2 DIRECT TENDERS
22.3 RETAIL SALES
22.3.1 ONLINE SALES
22.3.2 OFFLINE SALES
22.4 OTHERS
23 EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: EUROPE
23.2 MERGERS & ACQUISITIONS
23.3 NEW PRODUCT DEVELOPMENT & APPROVALS
23.4 EXPANSIONS
23.5 REGULATORY CHANGES
23.6 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET, BY REGION
24.1 EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.1.1 EUROPE
24.1.1.1. GERMANY
24.1.1.2. FRANCE
24.1.1.3. U.K.
24.1.1.4. ITALY
24.1.1.5. SPAIN
24.1.1.6. RUSSIA
24.1.1.7. TURKEY
24.1.1.8. BELGIUM
24.1.1.9. NETHERLANDS
24.1.1.10. SWITZERLAND
24.1.1.11. REST OF EUROPE
24.1.2 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 EUROPE AESTHETIC ENERGY-BASED DEVICE MARKET, COMPANY PROFILE
(SWOT AND DBMR ANALYSIS OF TOP 5 COMPANIES WILL BE PROVIDED)
25.1 CLASSYS INC
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPEMENTS
25.2 ALMA LASERS LTD.
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPEMENTS
25.3 CANDELA CORPORATION
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPEMENTS
25.4 MERZ PHARMA
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPEMENTS
25.5 AESTHETICS BIOMEDICAL
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPEMENTS
25.6 CUTERA INC.
25.6.1 COMPANY OVERVIEW
25.6.2 GEOGRAPHIC PRESENCE
25.6.3 PRODUCT PORTFOLIO
25.6.4 RECENT DEVELOPEMENTS
25.7 HOLOGIC INC.
25.7.1 COMPANY OVERVIEW
25.7.2 GEOGRAPHIC PRESENCE
25.7.3 PRODUCT PORTFOLIO
25.7.4 RECENT DEVELOPEMENTS
25.8 SCITON INC.
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPEMENTS
25.9 BAUSCH HEALTH COMPANIES INC.
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPEMENTS
25.1 LUMENIS BE LTD.
25.10.1 COMPANY OVERVIEW
25.10.2 GEOGRAPHIC PRESENCE
25.10.3 PRODUCT PORTFOLIO
25.10.4 RECENT DEVELOPEMENTS
25.11 S VENUS CONCEPT LTD.
25.11.1 COMPANY OVERVIEW
25.11.2 GEOGRAPHIC PRESENCE
25.11.3 PRODUCT PORTFOLIO
25.11.4 RECENT DEVELOPEMENTS
25.12 SHENZHEN GSD TECH CO., LTD.
25.12.1 COMPANY OVERVIEW
25.12.2 GEOGRAPHIC PRESENCE
25.12.3 PRODUCT PORTFOLIO
25.12.4 RECENT DEVELOPEMENTS
25.13 DEKA + CARTESSA AESTHETICS
25.13.1 COMPANY OVERVIEW
25.13.2 GEOGRAPHIC PRESENCE
25.13.3 PRODUCT PORTFOLIO
25.13.4 RECENT DEVELOPEMENTS
25.14 EL.EN. S.P.A.
25.14.1 COMPANY OVERVIEW
25.14.2 GEOGRAPHIC PRESENCE
25.14.3 PRODUCT PORTFOLIO
25.14.4 RECENT DEVELOPEMENTS
25.15 TRIA BEAUTY INC.
25.15.1 COMPANY OVERVIEW
25.15.2 REVENUE ANALYSIS
25.15.3 GEOGRAPHIC PRESENCE
25.15.4 PRODUCT PORTFOLIO
25.15.5 RECENT DEVELOPEMENTS
25.16 FOTONA
25.16.1 COMPANY OVERVIEW
25.16.2 GEOGRAPHIC PRESENCE
25.16.3 PRODUCT PORTFOLIO
25.16.4 RECENT DEVELOPEMENTS
25.17 LUTRONIC
25.17.1 COMPANY OVERVIEW
25.17.2 GEOGRAPHIC PRESENCE
25.17.3 PRODUCT PORTFOLIO
25.17.4 RECENT DEVELOPEMENTS
25.18 SHARPLIGHT TECHNOLOGIES INC.
25.18.1 COMPANY OVERVIEW
25.18.2 GEOGRAPHIC PRESENCE
25.18.3 PRODUCT PORTFOLIO
25.18.4 RECENT DEVELOPEMENTS
25.19 SINCLAIR
25.19.1 COMPANY OVERVIEW
25.19.2 GEOGRAPHIC PRESENCE
25.19.3 PRODUCT PORTFOLIO
25.19.4 RECENT DEVELOPEMENTS
25.2 CUTIS MEDICAL
25.20.1 COMPANY OVERVIEW
25.20.2 GEOGRAPHIC PRESENCE
25.20.3 PRODUCT PORTFOLIO
25.20.4 RECENT DEVELOPEMENTS
25.21 AEROLASE CORP.
25.21.1 COMPANY OVERVIEW
25.21.2 GEOGRAPHIC PRESENCE
25.21.3 PRODUCT PORTFOLIO
25.21.4 RECENT DEVELOPEMENTS
26 RELATED REPORTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.