Europe Balloon Catheter Market
Market Size in USD Billion
CAGR :
% 
 USD
1.26 Billion
USD
1.97 Billion
2024
2032
USD
1.26 Billion
USD
1.97 Billion
2024
2032
| 2025 –2032 | |
| USD 1.26 Billion | |
| USD 1.97 Billion | |
|
|
|
|
Europe Balloon Catheter Market Size
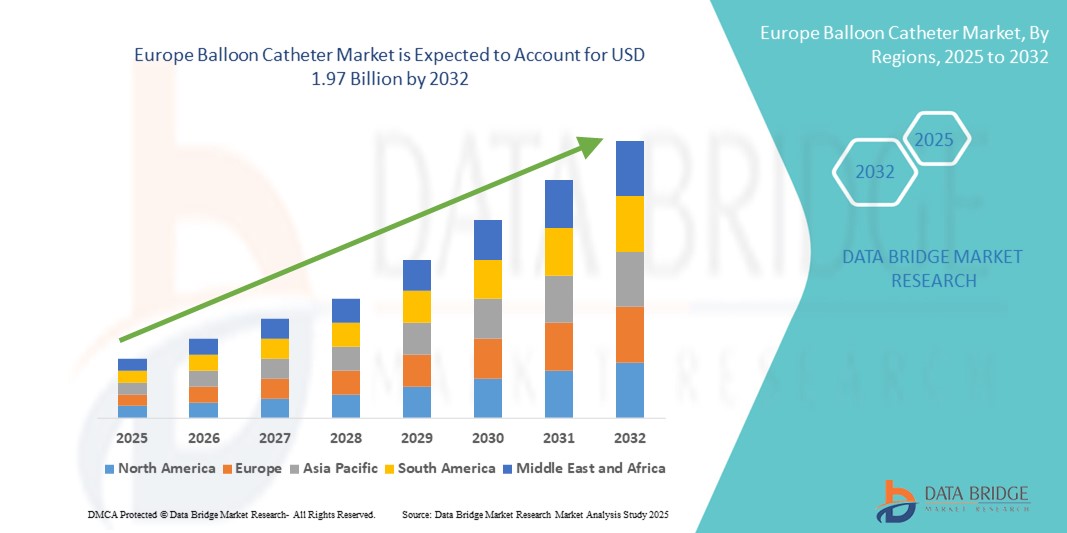
- The Europe balloon catheter market size was valued at USD 1.26 billion in 2024 and is expected to reach USD 1.97 billion by 2032, at a CAGR of 5.70% during the forecast period
- The market growth is primarily driven by the rising prevalence of cardiovascular and urological disorders, along with a growing aging population that necessitates minimally invasive treatment options across the region
- In addition, technological innovations such as drug-coated and dual-lumen balloon catheters are enhancing procedural efficacy and safety, thereby increasing adoption rates among healthcare professionals. These dynamics are positioning balloon catheters as vital tools in modern interventional procedures, contributing significantly to market expansion across Europe
Europe Balloon Catheter Market Analysis
- Balloon catheters, used for minimally invasive procedures such as angioplasty and vessel dilation, are increasingly vital components of interventional cardiology and radiology in both public and private healthcare institutions across Europe due to their precision, reduced recovery time, and high procedural success rates
- The demand for balloon catheters is primarily driven by the rising prevalence of cardiovascular diseases, aging population, and a shift toward non-surgical treatment options across key European countries
- Germany dominated the balloon catheter market with the largest revenue share of 29.5% in 2024, supported by a strong healthcare infrastructure, high interventional procedure volumes, and robust medical device innovation
- Poland is expected to be the fastest-growing country in the Europe balloon catheter market during the forecast period, due to increased government investment in healthcare modernization, rising awareness of early-stage treatment, and expansion of cardiovascular care facilities
- The PTCA balloon catheter segment dominated the Europe balloon catheter market with a market share of 52.9% in 2024, driven by its widespread use in treating coronary artery disease and its effectiveness in opening blocked arteries through percutaneous coronary interventions
Report Scope and Europe Balloon Catheter Market Segmentation
|
Attributes |
Europe Balloon Catheter Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Europe Balloon Catheter Market Trends
Technological Advancements Driving Precision and Therapeutic Outcomes
- A key and accelerating trend in the Europe balloon catheter market is the integration of next-generation technologies that improve procedural precision and enhance clinical outcomes, particularly in cardiovascular and peripheral interventions
- For instance, companies such as Medtronic and BIOTRONIK are introducing drug-coated balloon (DCB) catheters with innovative drug delivery mechanisms and anti-restenotic coatings that improve vascular healing and reduce the need for repeat interventions
- In addition, the trend toward image-guided and pressure-sensitive balloon catheters is enabling interventional cardiologists to make real-time procedural adjustments, improving safety and effectiveness in complex lesions
- The development of semi-compliant and high-pressure balloons has expanded the use of balloon catheters in heavily calcified and tortuous vessels, areas where traditional options were less effective. These advancements are particularly critical in aging populations prone to advanced atherosclerosis
- Innovations such as dual-lumen microcatheters are also enabling access to small or complex anatomies, supporting the growth of minimally invasive techniques across European catheterization labs
- As healthcare providers seek value-based technologies, manufacturers are focusing on enhancing catheter durability, reducing procedural times, and improving patient outcomes. Companies such as Boston Scientific and Terumo are investing in R&D to develop versatile balloon catheter platforms with customizable features to suit varied clinical needs
- These innovations are reshaping practitioner expectations, driving adoption in both academic and private cardiology centers, and reinforcing balloon catheters as a cornerstone of modern vascular therapy across Europe
Europe Balloon Catheter Market Dynamics
Driver
Rising Cardiovascular Burden and Shift to Minimally Invasive Interventions
- The growing incidence of cardiovascular diseases, particularly coronary artery disease (CAD) and peripheral artery disease (PAD), is a major driver of balloon catheter demand across Europe. Aging populations in countries such as Germany, Italy, and the UK are particularly affected
- For instance, the European Society of Cardiology reports that cardiovascular disease accounts for over 3.9 million deaths annually in Europe, prompting increased investments in interventional cardiology infrastructure
- As patients and healthcare systems favor minimally invasive procedures with quicker recovery times and fewer complications, balloon catheters are gaining prominence as frontline tools in endovascular therapy
- Hospitals and specialty centers across the region are expanding catheterization labs and adopting latest-generation DCBs and PTCA catheters, further accelerating market growth
- In addition, supportive reimbursement policies and early adoption of innovative medical devices in countries such as Germany and France are facilitating greater usage of balloon catheter technologies in routine clinical practice
Restraint/Challenge
Regulatory Complexity and Cost Constraints in Emerging Markets
- One of the primary challenges facing the Europe balloon catheter market is the complex and evolving regulatory environment, particularly under the European Union’s Medical Device Regulation (MDR) framework. The stringent compliance and certification requirements have increased time-to-market for newer devices and placed added pressure on manufacturers
- Smaller players face difficulties navigating these regulations, often resulting in limited product availability or delays in innovation rollout. For instance, re-certification of legacy products under MDR has strained the resources of many mid-sized European medtech firms
- In addition, the high cost of advanced balloon catheter systems, especially DCBs and cutting balloons, can hinder widespread adoption in price-sensitive healthcare systems such as those in Eastern Europe. Hospitals operating under strict public budgets may delay the procurement of high-end devices unless backed by strong evidence of cost-effectiveness
- Tackling these barriers through streamlined regulatory processes, pan-European clinical trials, and strategic pricing models will be essential for ensuring balanced market growth and greater access to life-saving interventions across the region
Europe Balloon Catheter Market Scope
The market is segmented on the basis of type, product type, delivery platform, compliance, balloon material, balloon type, application, end user, and distribution channel.
- By Type
On the basis of type, the Europe balloon catheter market is segmented into PTCA balloon catheters, CTO balloon catheters, and microcatheters. The PTCA balloon catheter segment dominated the market with the largest market revenue share of 52.9% in 2024, driven by its widespread use in percutaneous coronary interventions (PCI) for coronary artery disease. Clinicians prefer PTCA catheters due to their proven efficacy, broad availability, and compatibility with modern stenting procedures. The segment also benefits from increased procedural volumes in countries with aging populations and high cardiovascular risk.
The CTO balloon catheter segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by growing awareness and diagnosis of chronic total occlusions and the demand for specialized solutions in complex coronary anatomy. Their advanced deliverability and ability to access fully occluded vessels contribute to their growing role in interventional cardiology.
- By Product Type
On the basis of product type, the Europe balloon catheter market is segmented into normal balloon catheter, drug eluting balloon catheter, cutting balloon catheter, stent graft balloon catheter, and scoring balloon catheter. The drug eluting balloon catheter segment dominated the market with the largest market revenue share of 39.7% in 2024, attributed to its ability to provide local drug delivery without the need for permanent implants. Clinicians favor DEBs for their effectiveness in restenosis prevention and reduced late thrombosis risk, particularly in small vessel disease and in-stent restenosis.
The scoring balloon catheter segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by its enhanced plaque modification capabilities, particularly in fibrotic and calcified lesions. Its precision and lower risk of dissection support growing adoption in complex PCI procedures.
- By Delivery Platform
On the basis of delivery platform, the Europe balloon catheter market is segmented into rapid exchange (RX) / monorail balloon catheter, over-the-wire (OTW), and fixed wire (FW) balloon catheter. The rapid exchange (RX) segment dominated the market with the largest market revenue share of 46.1% in 2024, owing to its simplified single-operator use, reduced procedure time, and compatibility with modern interventional systems. RX catheters are especially popular in high-volume cardiology centers due to their ease of tracking and wire management.
The over-the-wire (OTW) segment is anticipated to witness the fastest growth rate from 2025 to 2032, supported by its superior performance in long and tortuous lesions and its essential role in peripheral procedures where additional guidewire support is required.
- By Compliance
On the basis of compliance, the Europe balloon catheter market is segmented into non-compliant, semi-compliant, and compliant balloons. The semi-compliant segment dominated the market with the largest market revenue share of 43.8% in 2024, due to its versatile nature offering both flexibility and moderate pressure, making it ideal for vessel preparation and post-dilation procedures.
The non-compliant segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by its use in high-pressure inflation and precision sizing, particularly in calcified lesions and stent post-dilation where radial strength is critical.
- By Balloon Material
On the basis of balloon material, the Europe balloon catheter market is segmented into nylon, polyethylene terephthalate (PET), polyethylene (PE), silicone, polyolefin copolymer, and others. The polyethylene terephthalate (PET) segment dominated the market with the largest market revenue share of 35.6% in 2024, owing to its excellent tensile strength and low compliance, which enable precise vessel dilation and high-pressure inflation. PET is widely used in coronary and peripheral applications requiring balloon durability and trackability.
The polyolefin copolymer segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by its favorable elongation properties and growing adoption in newer catheter designs where flexibility and thinner profiles are needed.
- By Balloon Type
On the basis of balloon type, the Europe balloon catheter market is segmented into high-pressure balloons and elastomeric balloons. The high-pressure balloon segment dominated the market with the largest market revenue share of 59.4% in 2024, driven by its widespread use in lesion preparation, post-dilation, and stent optimization. High-pressure balloons are particularly valuable in managing calcified lesions where higher inflation forces are needed for effective plaque modification.
The elastomeric balloon segment is anticipated to witness the fastest growth rate from 2025 to 2032, supported by their use in gentle vessel dilation, pediatric procedures, and pressure-sensitive anatomical areas where lower burst pressure is beneficial.
- By Application
On the basis of application, the Europe balloon catheter market is segmented into coronary artery disease, peripheral artery disease, and others. The coronary artery disease segment dominated the market with the largest market revenue share of 61.2% in 2024, owing to high procedural volumes and increasing prevalence of ischemic heart conditions across Europe. The segment benefits from clinical guidelines favoring PCI and the availability of advanced imaging and diagnostic tools in developed nations.
The peripheral artery disease segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by the rising incidence of diabetes, smoking, and obesity in Europe, especially in Eastern countries, leading to greater demand for peripheral interventions.
- By End User
On the basis of end user, the Europe balloon catheter market is segmented into hospitals, specialty centers, ambulatory surgery centers, and others. The hospital segment dominated the market with the largest market revenue share of 66.5% in 2024, due to the presence of specialized staff, reimbursement coverage, and higher procedure volumes. Hospitals remain the primary setting for complex cardiac and vascular procedures across Europe.
The ambulatory surgery center segment is anticipated to witness the fastest growth rate from 2025 to 2032, supported by the rise in same-day minimally invasive interventions, cost-effectiveness, and advancements in compact catheter lab equipment.
- By Distribution Channel
On the basis of distribution channel, the Europe balloon catheter market is segmented into direct tender, third party distribution, and others. The direct tender segment dominated the market with the largest market revenue share of 53.9% in 2024, owing to centralized hospital procurement systems and long-term contracts with manufacturers that ensure consistent supply of devices.
The third-party distribution segment is anticipated to witness the fastest growth rate from 2025 to 2032, particularly in smaller healthcare facilities and private clinics, where flexible delivery models and local distributor relationships enable quicker access to specialized balloon catheter products.
Europe Balloon Catheter Market Regional Analysis
- Germany dominated the balloon catheter market with the largest revenue share of 29.5% in 2024, supported by a strong healthcare infrastructure, high interventional procedure volumes, and robust medical device innovation
- The country’s emphasis on minimally invasive cardiovascular treatments, combined with robust reimbursement systems and well-established clinical practices, makes it a key hub for both innovation and adoption of balloon catheter technologies
- In addition, Germany’s aging population and rising incidence of coronary and peripheral artery diseases are fueling the demand for efficient endovascular interventions, reinforcing its leadership position within the European market
The Germany Balloon Catheter Market Insight
The Germany balloon catheter market captured the largest revenue share within Europe in 2024, owing to the country’s strong clinical adoption of interventional procedures and high investment in healthcare innovation. Germany’s advanced hospital infrastructure, skilled medical professionals, and early adoption of cutting-edge balloon catheter technologies, such as drug-coated and scoring balloons, drive market performance. Furthermore, the growing demand for endovascular treatment of chronic total occlusion and peripheral artery disease further supports market expansion, positioning Germany as a dominant force in the European balloon catheter space.
U.K. Balloon Catheter Market Insight
The U.K. balloon catheter market is poised for considerable growth over the forecast period, driven by the country’s focus on reducing invasive surgical procedures and expanding use of outpatient cardiac care. The increasing number of percutaneous coronary interventions (PCI), particularly in urban hospitals and NHS specialty centers, is bolstering demand for efficient balloon catheters. In addition, government support for early diagnosis and management of cardiovascular diseases, along with access to advanced therapeutic technologies, supports the market’s positive outlook in the U.K.
France Balloon Catheter Market Insight
The France balloon catheter market is projected to expand steadily during the forecast period, supported by a growing aging population, high awareness of cardiovascular health, and increasing prevalence of peripheral artery disease. Public-private partnerships promoting healthcare innovation and modern hospital infrastructures enable quick adoption of advanced catheter-based procedures. In addition, initiatives to reduce surgical burden through minimally invasive techniques are expected to strengthen demand for specialized balloon catheters across hospitals and ambulatory surgery centers in the country.
Italy Balloon Catheter Market Insight
The Italy balloon catheter market is experiencing moderate growth during the forecast period, fueled by the increasing use of drug-eluting and cutting balloon catheters in coronary and peripheral interventions. The Italian healthcare system’s investment in cardiovascular care, coupled with physician preference for catheter-based minimally invasive procedures, drives market expansion. Furthermore, growing collaborations between domestic and international medical device firms are contributing to improved accessibility and innovation in catheter technologies across Italy.
Poland Balloon Catheter Market Insight
The Poland balloon catheter market is witnessing growing momentum during the forecast period, supported by significant improvements in healthcare infrastructure and increasing adoption of interventional cardiology techniques. Rising incidence of cardiovascular conditions and government efforts to modernize cardiac care services are driving demand for balloon catheters in both public and private hospitals. In addition, Poland’s integration into EU healthcare initiatives and increased access to advanced medical devices are contributing to broader adoption of high-performance balloon catheters for coronary and peripheral procedures.
Europe Balloon Catheter Market Share
The Europe balloon catheter industry is primarily led by well-established companies, including:
- Medtronic (Ireland)
- Boston Scientific Corporation (U.S.)
- Abbott Laboratories (U.S.)
- BIOTRONIK SE & Co. KG (Germany)
- B. Braun SE (Germany)
- Terumo Corporation (Japan)
- Cordis Corporation (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- Cook (U.S.)
- MicroPort Scientific Corporation (China)
- Lepu Medical Technology (Beijing) Co., Ltd. (China)
- Acrostak AG (Switzerland)
- Nipro Corporation (Japan)
- Balton Sp. z o.o. (Poland)
- Meril Life Sciences Pvt. Ltd. (India)
- iVascular S.L.U. (Spain)
- Hexacath France (France)
- AngioDynamics, Inc. (U.S.)
- Cardionovum GmbH (Germany)
- Alvimedica Medical Technologies Inc. (Turkey)
What are the Recent Developments in Europe Balloon Catheter Market?
- In June 2024, BIOTRONIK SE & Co. KG, a leading German-based medical device company, launched its latest generation of drug-coated balloon catheters—designed specifically for peripheral artery disease treatment across Europe. The innovation focuses on enhanced drug delivery efficiency and vessel healing, reinforcing BIOTRONIK’s commitment to clinical excellence and patient-centered technology. This strategic development further cements the company’s leadership in the European interventional cardiology market
- In May 2024, Medtronic plc introduced its cutting-edge scoring balloon catheter platform in the United Kingdom, aimed at improving outcomes in complex coronary interventions. The product’s enhanced lesion modification capabilities are designed for highly calcified and resistant plaques. This launch signifies Medtronic’s strategic investment in expanding its cardiovascular solutions portfolio tailored to the evolving needs of European clinicians
- In March 2024, Terumo Europe N.V. announced the expansion of its manufacturing facility in Belgium to increase production capacity for its microcatheter and PTCA balloon catheter lines. This move supports the growing demand for minimally invasive cardiovascular treatments in Europe and showcases Terumo’s commitment to delivering reliable and high-performance interventional tools across regional healthcare markets
- In February 2024, B. Braun Melsungen AG unveiled a new line of semi-compliant balloon catheters under its SeQuent family, targeting improved precision in peripheral procedures. Launched in France and Italy, the catheter is designed to offer enhanced flexibility and pushability, aligning with the growing trend of personalized, patient-specific treatment plans in interventional radiology and vascular surgery
- In January 2024, Cardinal Health expanded its strategic distribution agreement with European hospitals for its drug-eluting balloon catheter range, including countries such as Spain, Germany, and Poland. The agreement aims to streamline access to advanced balloon technologies, enhancing procedural efficiency and patient care across diverse healthcare settings. This collaboration highlights Cardinal Health’s focus on expanding its global footprint and strengthening service delivery in the European interventional market
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.