Europe Cancer Diagnostics Market
Market Size in USD Million
CAGR :
% 
 USD
18,369.60 Million
USD
31,093.51 Million
2022
2030
USD
18,369.60 Million
USD
31,093.51 Million
2022
2030
| 2023 –2030 | |
| USD 18,369.60 Million | |
| USD 31,093.51 Million | |
|
|
|
|
Europe Cancer Diagnostics Market Analysis and Size
Advances in cancer diagnoses and therapy have resulted in a large increase in survival rates, particularly among children with cancer, resulting in a paradigm change in attention toward long-term survivorship and quality-of-life issues. While cardiac, pulmonary, renal, and hepatic functions are routinely evaluated before, during, and after treatment, the reproductive system is not. As more girls and women survive cancer, it's more important than ever to predict the effects of cancer and its treatment and provide options to protect this population's reproductive and sexual function.
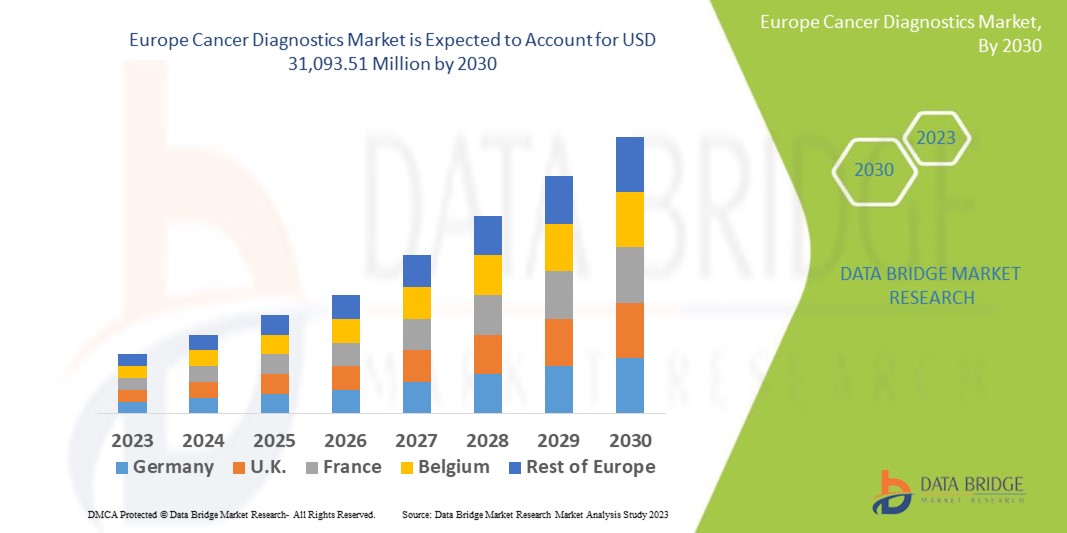
Data Bridge Market Research analyses that the Europe cancer diagnostics market, which was USD 18,369.60 million in 2022, is expected to reach USD 31,093.51 million by 2030, and is expected to undergo a CAGR of 6.80% during the forecast period. The instruments dominates the product type segment of the cancer diagnostics market owing to the advancements in technology. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Europe Cancer Diagnostics Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product Type (Instrument, Consumables and Accessories, Software and Service), Test Type (Imaging, Biopsy, Tumor Biomarkers Tests, Genetic Tests, Endoscopy, Immunohistochemistry, Others), Technology (Instrument Based, Platform-Based, Tumor Biomarker Tests), Application (Breast Cancer, Prostate Cancer, Colorectal Cancer, Lung Cancer, Skin Cancer, Kidney Cancer, Blood Cancer, Pancreatic Cancer, Ovarian Cancer, Cervical Cancer, Others), Age Group (Adult, Pediatric), End-User (Hospitals, Oncology Speciality Clinics, Diagnostic Laboratories, Cancer Research Institutes, Others), Distribution Channel (Direct tenders, Retail Sales) |
|
Countries Covered |
Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe |
|
Market Players Covered |
General Electric (U.S.), Abbott (U.S.), Hologic Inc. (U.S.), Agilent Technologies Inc. (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), FUJIFILM Corporation (Japan), Danaher (U.S.), DiaSorin S.P.A. (Italy), Myriad Genetics Inc. (U.S.), Siemens Healthcare GmbH (Germany), BD (U.S.), bioMérieux (France), Bio-Rad Laboratories Inc. (U.S.), Cancer Diagnostics Inc. (U.S.), Vela Diagnostics (Singapore), AMOY Diagnostics CO. LTD. (China), Quidel Corporation (U.S.), Bio SB (U.S.), Biocartis (Belgium), Exact Science (U.S.) |
|
Market Opportunities |
|
Market Definition
Cancer is a disease in which some cells develop uncontrollably and spread throughout the body. In the millions of cells that make up the human body, cancer can start almost anywhere. Human cells expand and proliferate (through a process known as cell division) to produce new cells that the body requires. As cells age or become harmed, they die and are replaced by new cells.
Europe Cancer Diagnostics Market Dynamics
Drivers
- Rising Demand for Early and Accurate Disease Diagnosis
Increasing healthcare spending on cancer treatment offers an opportunity to drive market demand. A growing senior population which is more susceptible to infectious diseases is driving the market.
- Growth in the Number of Private Diagnostic Centers
The number of private diagnostic centres is growing around the world as demand for diagnostic imaging treatments rises and public hospitals struggle to keep up with the restricted number of imaging modalities at their disposal. FUJIFILM Corporation opened NURA, a medical screening centre specializing in cancer screening, in Bangalore, India. This medical screening centre will be administered by FUJIFILM DKH LLP (FUJIFILM DKH) and Dr. Kutty's Healthcare in January 2021. (DKH). FUJIFILM DKH LLP (FUJIFILM DKH) is a joint project between FUJIFILM and Dr. Kutty's Healthcare (DKH), a hospital and screening facility owner and operator in India and the Middle East.
- Upsurge in the Emergence of New Technologies
Biomarkers and point-of-care testing, a preference for sophisticated, less-painful, and far more efficient tests over traditional tests, increasing healthcare spending, increased awareness, and increased government backing are driving the cancer diagnostics industry forward.
Opportunities
- Growing Demand for Personalized Medicine
Personalized medicine, which involves tailoring treatment plans based on an individual's genetic makeup, is gaining traction in Europe. This approach requires accurate and comprehensive cancer diagnostics to identify specific mutations and biomarkers, driving the demand for advanced diagnostic tests.
- Collaborations and Partnerships
Increasing collaborations between diagnostic companies, research institutions, and healthcare providers have accelerated the development and adoption of innovative cancer diagnostic technologies. Such partnerships facilitate knowledge sharing, technology transfer, and market expansion, further driving the growth of the cancer diagnostic market in Europe.
Restraints/Challenges
- Stringent Regulatory Requirements
Europe has strict regulations in place for the approval and commercialization of diagnostic tests. This can create significant barriers for companies trying to enter the market, leading to delays in product launches and increased costs.
- Limited Reimbursement Policies
Reimbursement policies for cancer diagnostic tests vary across European countries. In some cases, the reimbursement rates may not adequately cover the costs of the tests, which can limit their adoption and accessibility
This cancer diagnostics market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the cancer diagnostics market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In March 2020, Siemens Healthineers AG stated that their AI-Pathway Companion Prostate Cancer, a digital companion utilized in the clinical pathway of prostate cancer, has achieved CE mark approval. The company began selling their product throughout Europe after receiving authorization
- In November 2021, Siemens Healthineers introduced the Naeotom Alpha, the world's first photon-counting CT scanner with increased resolution and up to a 45 percent reduction in radiation exposure for ultra-high resolution scans. As a result of the causes above, the segment is likely to increase in the near future
Europe Cancer Diagnostics Market Scope
The cancer diagnostics market is segmented on the basis of product type, test type, technology, application, age group, distribution channel and end-user. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Instrument
- Consumables and Accessories
- Software and Service
Test Type
- Diagnostic Imaging Tests
- Biopsy and Cytology Tests
- Tumor Biomarkers
- Other Diagnostic Types
Technology
- Instrument Based
- Platform-Based
- Tumor Biomarker Tests
Distribution Channel
- Direct tenders
- Retail Sales
Application
- Breast Cancer
- Lung Cancer
- Cervical Cancer
- Kidney Cancer
- Liver Cancer
- Pancreatic Cancer
- Ovarian Cancer
- Other Applications
Age Group
- Adult
- Pediatric
End-User
- Hospital
- Oncology Speciality Clinics
- Diagnostic Laboratories
- Cancer Research Institutes
- Others
Europe Cancer Diagnostics Market Regional Analysis/Insights
The cancer diagnostics market is analysed and market size insights and trends are provided by product type, test type, technology, application, age group, distribution channel and end-user as referenced above.
The countries covered in the cancer diagnostics market report are Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, and Rest of Europe in Europe.
Germany has the largest market share, owing to the high prevalence of cancer among the population and the widespread availability of diagnostic systems and consumables in the country.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and up-stream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of regional brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The cancer diagnostics market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for cancer diagnostics market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the cancer diagnostics market. The data is available for historic period 2015 to 2020.
Competitive Landscape and Europe Cancer Diagnostics Market Share Analysis
The cancer diagnostics market competitive landscape provides details by competitor details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, regional presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance the above data points provided are only related to the company's focus related to cancer diagnostics market.
Some of the major players operating in the cancer diagnostics market are:
- General Electric (U.S.)
- Abbott (U.S.)
- Hologic Inc. (U.S.)
- Agilent Technologies Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- FUJIFILM Corporation (Japan)
- Danaher (U.S.)
- DiaSorin S.P.A. (Italy)
- Myriad Genetics Inc. (U.S.)
- Siemens Healthcare GmbH (Germany)
- BD (US), bioMérieux (France)
- Bio-Rad Laboratories Inc. (U.S.)
- Cancer Diagnostics Inc. (U.S.)
- Vela Diagnostics (Singapore)
- AMOY Diagnostics CO. LTD. (China)
- Quidel Corporation (U.S.)
- Bio SB (U.S.)
- Biocartis (Belgium)
- Exact Science (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.