Europe Drug Safety Solutions And Pharmacovigilance Market
Market Size in USD Million
CAGR :
% 
 USD
57.06 Million
USD
98.78 Million
2024
2032
USD
57.06 Million
USD
98.78 Million
2024
2032
| 2025 –2032 | |
| USD 57.06 Million | |
| USD 98.78 Million | |
|
|
|
|
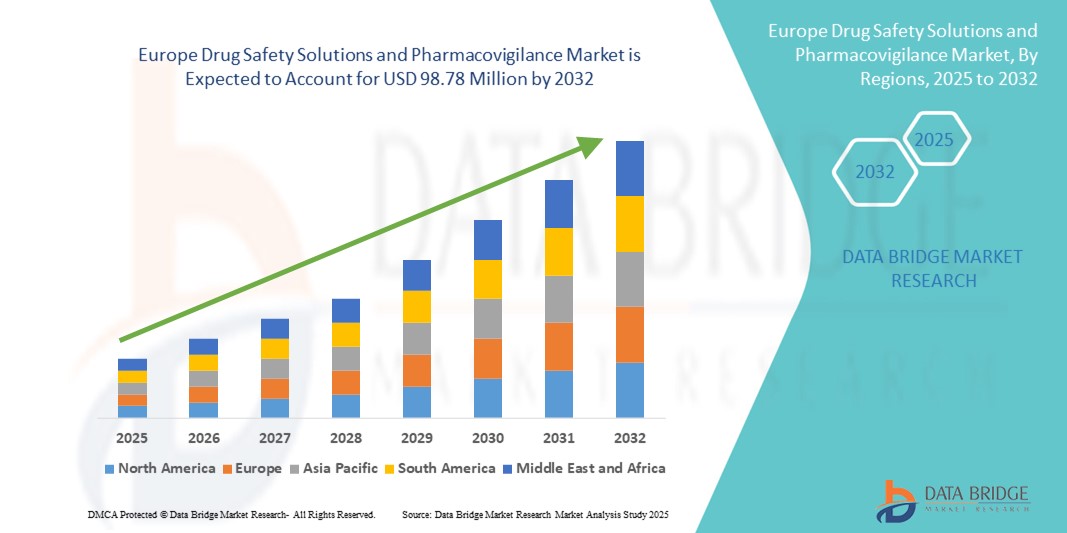
Europe Drug Safety Solutions and Pharmacovigilance Market Size
- The Europe drug safety solutions and pharmacovigilance market size was valued at USD 57.06 million in 2024 and is expected to reach USD 98.78 million by 2032, at a CAGR of 7.10% during the forecast period
- The market growth is largely fueled by stringent regulatory requirements such as the EU Pharmacovigilance Directive, increasing healthcare expenditures, expanding clinical research activities, and heightened awareness of drug safety practices across the region
- Furthermore, rising demand from pharmaceutical companies and contract research organizations for robust, user-friendly, and integrated drug safety and pharmacovigilance solutions is establishing these platforms as essential tools for monitoring adverse events and ensuring patient safety. These converging factors are accelerating the adoption of advanced pharmacovigilance systems, thereby significantly boosting the industry's growth
Europe Drug Safety Solutions and Pharmacovigilance Market Analysis
- Drug safety solutions and pharmacovigilance platforms, providing comprehensive monitoring, reporting, and management of adverse drug reactions, are increasingly vital components of modern pharmaceutical operations and healthcare systems in both clinical and post-marketing settings due to their critical role in ensuring patient safety, regulatory compliance, and data integrity
- The escalating demand for pharmacovigilance solutions is primarily fueled by stringent regulatory requirements in the EU, increasing clinical trial activities, rising drug development complexity, and growing awareness of patient safety and drug risk management
- Germany dominated the Europe drug safety solutions and pharmacovigilance market with the largest revenue share of 39% in 2024, driven by its high healthcare expenditure, strong presence of pharmaceutical companies, and well-established contract research organizations actively adopting advanced safety monitoring systems
- France is expected to be the fastest-growing country in the Europe drug safety solutions and pharmacovigilance market during the forecast period, due to expanding clinical research infrastructure, rising healthcare investments, and increasing adoption of digital pharmacovigilance tools
- Adverse Event Reporting Software (AERS) segment dominated the Europe drug safety solutions and pharmacovigilance market with a market share of 43.5% in 2024, driven by its essential role in capturing, processing, and analyzing safety data efficiently across both clinical trials and post-marketing surveillance
Report Scope and Europe Drug Safety Solutions and Pharmacovigilance Market Segmentation
|
Attributes |
Europe Drug Safety Solutions and Pharmacovigilance Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Europe Drug Safety Solutions and Pharmacovigilance Market Trends
Enhanced Efficiency Through AI and Cloud-Based Platforms
- A significant and accelerating trend in the Europe drug safety solutions and pharmacovigilance market is the increasing integration of artificial intelligence (AI) and cloud-based platforms into safety monitoring and adverse event reporting systems. This integration is significantly enhancing operational efficiency, data accuracy, and regulatory compliance
- For instance, AI-enabled pharmacovigilance platforms can automatically detect and categorize adverse drug reactions from multiple data sources, reducing manual workload and accelerating safety reporting timelines. Cloud-based solutions allow seamless access to real-time safety data across multiple departments and sites
- AI integration enables predictive safety analytics, signal detection, and intelligent prioritization of high-risk adverse events, while cloud platforms facilitate centralized management of safety cases, audits, and regulatory submissions. This combination allows pharmaceutical companies to respond more proactively to safety concerns
- The seamless integration of pharmacovigilance systems with electronic health records (EHRs), clinical trial management systems (CTMS), and regulatory reporting portals creates a unified platform for end-to-end drug safety management, enhancing compliance and operational efficiency
- This trend towards more intelligent, automated, and interconnected pharmacovigilance systems is reshaping industry expectations for drug safety monitoring. Consequently, companies such as ArisGlobal and Oracle Health Sciences are developing AI-enabled solutions with automated case processing, signal detection, and cloud-based reporting capabilities
- The demand for AI-driven and cloud-integrated pharmacovigilance solutions is growing rapidly across pharmaceutical companies and contract research organizations, as regulators and stakeholders increasingly prioritize patient safety and timely safety reporting
Europe Drug Safety Solutions and Pharmacovigilance Market Dynamics
Driver
Rising Regulatory Requirements and Increased Clinical Research Activities
- The growing stringency of regulatory requirements in the EU, combined with an increase in clinical trials and post-marketing surveillance, is a significant driver for the heightened demand for pharmacovigilance solutions
- For instance, in 2024, several EU-based pharmaceutical companies adopted AI-enabled pharmacovigilance platforms to comply with updated EU Good Pharmacovigilance Practices (GVP) guidelines. Such proactive compliance strategies are expected to drive market growth in the forecast period
- As regulators enforce more rigorous safety reporting standards, drug safety platforms that provide automated reporting, case tracking, and real-time dashboards are becoming essential tools for pharmaceutical companies
- Furthermore, the increasing complexity of drug pipelines, biologics, and combination therapies is driving the adoption of advanced pharmacovigilance solutions capable of managing large volumes of safety data efficiently
- Centralized, cloud-based platforms that facilitate collaboration across clinical, regulatory, and safety departments enhance the overall efficiency and accuracy of drug safety management, further propelling market adoption
Restraint/Challenge
Data Privacy Concerns and High Implementation Costs
- Concerns surrounding patient data privacy, GDPR compliance, and the security of cloud-based pharmacovigilance platforms pose significant challenges to broader market adoption. Sensitive patient information requires strict encryption, access controls, and secure data storage to maintain trust and comply with regulations
- For instance, high-profile data breaches in healthcare IT systems have heightened awareness of potential risks, making some companies cautious in adopting new digital pharmacovigilance solutions
- Addressing these challenges through robust cybersecurity measures, regular system audits, and employee training on compliance protocols is critical for building confidence among users. In addition, the high initial investment required for implementing advanced AI-driven and cloud-based pharmacovigilance systems can be a barrier, particularly for small and mid-sized pharmaceutical companies
- While modular and subscription-based solutions are helping reduce upfront costs, the perceived expense of sophisticated pharmacovigilance platforms can still hinder adoption, especially among cost-conscious organizations
- Overcoming these challenges through secure, scalable, and cost-effective solutions, alongside regulatory compliance support and user training, will be vital for sustained growth in the Europe drug safety solutions and pharmacovigilance market
Europe Drug Safety Solutions and Pharmacovigilance Market Scope
The market is segmented on the basis of type, product, functionality, delivery, end users, and distribution channel.
- By Type
On the basis of type, the Europe drug safety solutions and pharmacovigilance market is segmented into software and services. The software segment dominated the market with the largest revenue share of 57.4% in 2024, driven by the growing adoption of automated systems for adverse event reporting, signal detection, and regulatory compliance. These software solutions are increasingly preferred by pharmaceutical companies and contract research organizations (CROs) due to their ability to handle large volumes of safety data, ensure real-time monitoring, and integrate seamlessly with electronic health records (EHRs), clinical trial management systems (CTMS), and regulatory reporting portals.
The services segment is anticipated to witness the fastest growth rate of 11.8% from 2025 to 2032, fueled by the rising demand for outsourced pharmacovigilance activities, including case processing, safety audits, regulatory submission support, and signal management. This is particularly prominent among small and mid-sized pharmaceutical firms that may lack the infrastructure or expertise to manage pharmacovigilance internally, leading to increased reliance on third-party service providers.
- By Product
On the basis of product, the Europe drug safety solutions and pharmacovigilance market is segmented into standard form and customized form solutions. The standard form segment held the largest market revenue share in 2024, due to its wide applicability, ease of deployment, and compliance with common regulatory requirements across EU countries. Standardized solutions provide a consistent framework for reporting adverse events, simplifying training, and ensuring regulatory alignment.
The customized form segment is expected to witness faster growth from 2025 to 2032, driven by the increasing need for tailored pharmacovigilance workflows that align with the internal processes, clinical trial protocols, and data management systems of specific pharmaceutical and biotechnology companies. Customized solutions allow flexibility for complex drug portfolios, specialized therapeutic areas, and unique reporting requirements, which is becoming a key differentiator in the competitive landscape.
- By Functionality
On the basis of functionality, the Europe drug safety solutions and pharmacovigilance market is segmented into adverse event reporting software (AERS), drug safety audits software, and issue tracking software. The AERS segment dominated the market with a share of 43.5% in 2024, as it is critical for capturing, processing, and analyzing adverse drug reaction reports efficiently across clinical and post-marketing phases. AERS ensures compliance with EMA guidelines and facilitates timely safety reporting.
Issue tracking software is projected to register strong growth during forecast period, supporting organizations in monitoring, investigating, and resolving safety issues promptly, thus minimizing risks and ensuring adherence to European Medicines Agency (EMA) guidelines and other regulatory frameworks. This functionality supports proactive risk management and enhances the reliability of pharmacovigilance systems.
- By Delivery
On the basis of delivery, the Europe drug safety solutions and pharmacovigilance market is segmented into on-premise delivery mode and on-demand/cloud-based (SaaS) delivery mode. The on-demand/cloud-based segment dominated in 2024 with a revenue share of 52%, owing to benefits such as scalability, real-time updates, centralized data access, lower upfront investment, and the ability to support geographically distributed teams. Cloud-based platforms also facilitate easier software upgrades, integration with AI-driven analytics, and seamless regulatory reporting.
The on-premise segment continues to grow steadily during forecast period, preferred by organizations with stringent data security protocols, internal IT infrastructure, and strict compliance requirements that necessitate local hosting of sensitive pharmacovigilance data. They provide local hosting of sensitive pharmacovigilance data and enhanced control over compliance and internal workflows.
- By End Users
On the basis of end users, the Europe drug safety solutions and pharmacovigilance market is segmented into biotechnology and pharmaceutical companies, contract research organizations (CROs), hospitals, KPOs/BPOs, and healthcare providers. Biotechnology and pharmaceutical companies held the largest revenue share of 46% in 2024, driven by the high volume of drug safety data generated during clinical trials and post-marketing surveillance, as well as the critical need to comply with stringent EU pharmacovigilance regulations.
CROs are anticipated to witness the fastest growth due to the increasing outsourcing of pharmacovigilance tasks, rapid expansion of clinical trial activities, and adoption of AI and cloud-based monitoring solutions to manage safety data across multiple clients and countries. The expansion of clinical trials and adoption of AI and cloud-based solutions for managing safety data across multiple clients and countries are key drivers.
- By Distribution Channel
On the basis of distribution channel, the Europe drug safety solutions and pharmacovigilance market is segmented into direct sales and retail sales. The direct sales segment dominated in 2024, accounting for 65% of market revenue, as pharmaceutical and biotechnology companies prefer purchasing solutions directly from vendors to ensure customization, dedicated support, integration services, and compliance assurance. This ensures customization, dedicated support, integration services, and regulatory compliance assurance.
Retail sales, including resellers and third-party distributors, are growing steadily during forecast period, particularly for standardized software solutions adopted by smaller healthcare providers, emerging pharmaceutical firms, and CROs. The growth in retail sales is supported by subscription-based models, bundled offerings, and increased awareness of cost-effective pharmacovigilance solutions in the market. Growth is further supported by subscription-based models, bundled offerings, and the increasing awareness of cost-effective pharmacovigilance solutions.
Europe Drug Safety Solutions and Pharmacovigilance Market Regional Analysis
- Germany dominated the Europe drug safety solutions and pharmacovigilance market with the largest revenue share of 39% in 2024, driven by its high healthcare expenditure, strong presence of pharmaceutical companies, and well-established contract research organizations actively adopting advanced safety monitoring systems
- Companies in Germany are increasingly adopting advanced pharmacovigilance software and services to ensure compliance with EU Good Pharmacovigilance Practices (GVP) and other regulatory requirements. This adoption is further supported by the country’s strong research infrastructure, a high volume of clinical trials, and increasing focus on patient safety and risk management
- The widespread adoption of these solutions across Europe is supported by stringent regulatory oversight, increasing awareness of drug safety, and the growing complexity of drug development processes, making pharmacovigilance systems essential tools for ensuring compliance and patient safety in both clinical and post-marketing phases
The Germany Drug Safety Solutions and Pharmacovigilance Market Insight
The Germany drug safety solutions and pharmacovigilance market captured the largest revenue share of 39% in 2024, fueled by the country’s well-established pharmaceutical industry, strong regulatory framework, and increasing clinical trial activities. Pharmaceutical companies and contract research organizations (CROs) are prioritizing the adoption of AI-enabled and cloud-based pharmacovigilance solutions to ensure compliance with EU Good Pharmacovigilance Practices (GVP) and streamline adverse event reporting. The emphasis on patient safety, risk management, and integration with electronic health records (EHRs) and clinical trial management systems further supports market growth.
France Drug Safety Solutions and Pharmacovigilance Market Insight
The France drug safety solutions and pharmacovigilance market is projected to grow at a substantial CAGR during the forecast period, driven by rising healthcare investments, expanding clinical research infrastructure, and the increasing demand for automated drug safety monitoring. French pharmaceutical companies are adopting cloud-based pharmacovigilance platforms to improve operational efficiency, ensure regulatory compliance, and enable centralized monitoring of adverse events across multiple sites.
U.K. Drug Safety Solutions and Pharmacovigilance Market Insight
The U.K. drug safety solutions and pharmacovigilance market is anticipated to grow at a noteworthy CAGR, supported by the adoption of advanced pharmacovigilance software and services in both pharmaceutical companies and healthcare providers. The increasing focus on patient safety, stringent regulatory oversight, and the rising volume of clinical trials are encouraging the use of AI-driven case processing, signal detection, and cloud-based reporting platforms. Strong digital infrastructure and a robust contract research ecosystem are further driving adoption.
Italy Drug Safety Solutions and Pharmacovigilance Market Insight
The Italy drug safety solutions and pharmacovigilance market is expected to expand steadily during the forecast period, fueled by growing awareness of drug safety, government initiatives to enhance pharmacovigilance practices, and increasing outsourcing of safety monitoring activities to CROs. Adoption of standardized and customized software solutions for adverse event reporting and compliance management is gaining traction among pharmaceutical firms operating in Italy.
Spain Drug Safety Solutions and Pharmacovigilance Market Insight
The Spain drug safety solutions and pharmacovigilance market is witnessing growth due to the increasing number of clinical trials, rising healthcare expenditure, and the need for efficient adverse event management systems. Cloud-based pharmacovigilance platforms and outsourced services are being increasingly adopted to streamline reporting, ensure regulatory compliance, and manage safety data efficiently across multiple healthcare facilities and research organizations.
Europe Drug Safety Solutions and Pharmacovigilance Market Share
The Europe drug safety solutions and pharmacovigilance industry is primarily led by well-established companies, including:
- IQVIA (U.S.)
- Parexel International (MA) Corporation (U.S.)
- Syneos Health (U.S.)
- ICON plc (Ireland)
- Cognizant (U.S.)
- Accenture (Ireland)
- ArisGlobal (U.S.)
- Genpact (U.S.)
- Flex Databases (U.K.)
- Asphalion (Spain)
- Realtime-testing (U.K.)
- Biologit (Belgium)
- Eurocept Pharmaceuticals (Netherlands)
- Qinecsa (U.K.)
- Aixial Group (France)
- Shionogi BV (Netherlands)
- F. Hoffmann-La Roche Ltd (Switzerland)
- CSL (Australia)
What are the Recent Developments in Europe Drug Safety Solutions and Pharmacovigilance Market?
- In July 2025, the European Commission adopted an Implementing Regulation that updates the rules for pharmacovigilance activities. This regulation, which will take effect in early 2026, aims to clarify and strengthen pharmacovigilance while also reducing administrative burdens. Key updates include a requirement for marketing authorization holders to document only "major or critical" deviations in their Pharmacovigilance System Master File (PSMF) and a clarification of their obligations to use data from the EudraVigilance database for signal management
- In June 2025, the European Commission introduced the 2025 EU Pharma Package, which includes regulations for the authorization and supervision of medicinal products and rules for the European Medicines Agency. This package aims to streamline procedures and enhance the development of medicines within the EU
- In May 2025, a draft report highlighted the increasing role of Artificial Intelligence (AI) and Machine Learning (ML) in pharmacovigilance. The report outlines key steps for life sciences companies to implement AI in post-market safety activities, emphasizing the need for compliance with the EU AI Act and the evolving regulatory landscape in the U.S
- In August 2024, the European Medicines Agency (EMA) implemented the revision of Good Pharmacovigilance Practices (GVP) Module XVI. This update introduced Addendum II, providing detailed guidance on assessing the effectiveness of risk minimization measures through structured data sources and advanced search methods
- In July 2021, Asphalion, a leading European regulatory affairs consultancy, launched a new EU Local Contact Network to provide local pharmacovigilance support across EU countries. This initiative aimed to implement global solutions for the externalisation of Pharmacovigilance services, including the European Qualified Person for Pharmacovigilance (EU-QPPv) and Pharmacovigilance System Outsourcing
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.