Europe Exosome Therapeutic Market
Market Size in USD million
CAGR :
% 
 USD
19.07 million
USD
71.95 million
2024
2032
USD
19.07 million
USD
71.95 million
2024
2032
| 2025 –2032 | |
| USD 19.07 million | |
| USD 71.95 million | |
|
|
|
|
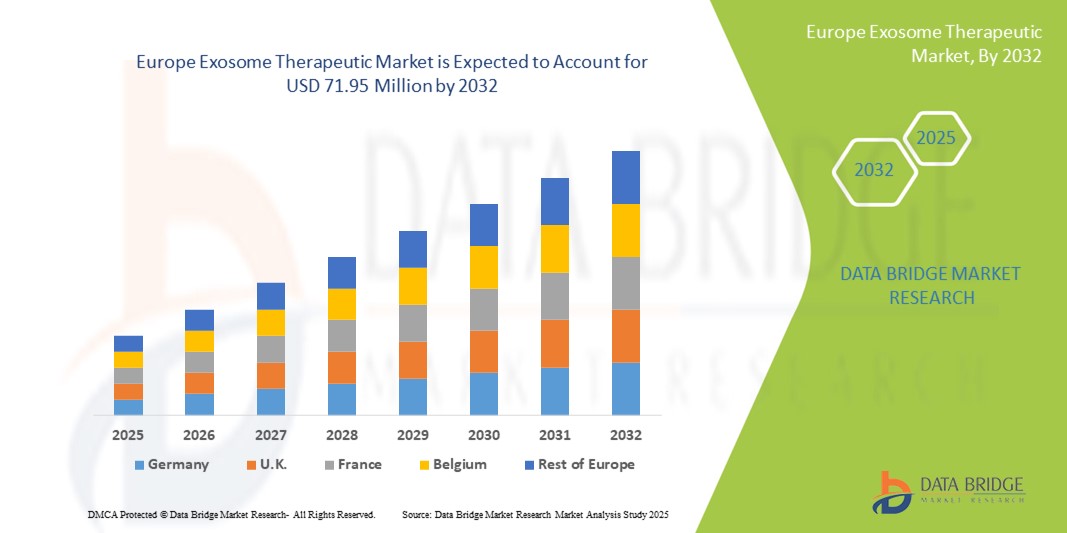
Europe Exosome Therapeutic Market Size
- The Europe exosome therapeutic market size was valued at USD 19.07 million in 2024 and is expected to reach USD 71.95 million by 2032, at a CAGR of 18.05% during the forecast period
- The market expansion is primarily driven by growing research activities and clinical trials exploring exosome-based therapies for oncology, neurodegenerative, cardiovascular, and inflammatory diseases, supported by favorable academic–industry collaborations across Europe
- In addition, increasing investments in precision medicine, coupled with strong regulatory support for advanced biologics, are fostering innovation and accelerating commercialization efforts, thereby positioning exosome therapeutics as a transformative treatment modality and fueling market growth
Europe Exosome Therapeutic Market Analysis
- Exosome therapeutics, leveraging naturally secreted vesicles for targeted drug delivery and regenerative medicine, are emerging as a transformative approach in treating oncology, neurodegenerative, cardiovascular, and inflammatory disorders, with growing relevance in both academic and commercial healthcare settings due to their precision, safety, and cell-free advantages
- The rising demand for exosome-based therapies is primarily fueled by increasing investments in advanced biologics, a surge in clinical trials, and expanding collaborations between biotech firms, pharmaceutical companies, and research institutes across Europe
- Germany dominated the exosome therapeutics market with the largest revenue share of 41.8% in 2024, supported by strong R&D infrastructure, favorable regulatory frameworks for advanced therapies, and significant funding programs from both the EU and national government, with German biotech clusters leading in translational research and commercialization efforts
- France is expected to be the fastest growing country in the exosome therapeutics market during the forecast period due to rising oncology-focused clinical trials, expanding biotech startup ecosystems, and government-backed initiatives to accelerate precision medicine adoption
- Oncology segment dominated the exosome therapeutics market with a market share of 45.1% in 2024, driven by the urgent need for novel, targeted treatment modalities, increasing cancer prevalence, and the strong focus of both clinical trials and commercial pipelines on exosome-based cancer therapeutics
Report Scope and Europe Exosome Therapeutic Market Segmentation
|
Attributes |
Europe Exosome Therapeutic Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Europe Exosome Therapeutic Market Trends
Expanding Applications in Oncology and Regenerative Medicine
- A significant and accelerating trend in the Europe exosome therapeutics market is the growing application of exosomes in oncology and regenerative medicine, supported by advancements in drug delivery and diagnostics technologies. This is enhancing treatment precision and therapeutic potential
- For instance, Exogenus Therapeutics in Portugal is advancing exosome-based regenerative solutions for wound healing, while Evox Therapeutics in the U.K. is developing exosome-based drug delivery platforms for genetic diseases and oncology
- Exosome platforms enable capabilities such as crossing the blood–brain barrier, targeted payload delivery, and reduced immunogenicity, making them attractive for novel therapeutic approaches. For instance, research from German biotech firms is leveraging exosomes to deliver RNA-based therapeutics for cancer and neurological disorders
- The seamless integration of exosome science with broader precision medicine initiatives is fostering collaboration between academia, biotech, and pharmaceutical companies across Europe. Through such partnerships, drug development pipelines are accelerating from preclinical to clinical phases
- This trend towards more advanced, clinically validated, and versatile exosome platforms is fundamentally reshaping the therapeutic landscape in Europe. Consequently, companies such as Codiak Biosciences and Exopharm are expanding their R&D collaborations in the region to strengthen translational progress
- The demand for exosome therapeutics that offer targeted, safe, and efficient treatment modalities is growing rapidly across oncology, neurology, and regenerative medicine, as patients and healthcare systems increasingly prioritize innovative and personalized solutions.
Europe Exosome Therapeutic Market Dynamics
Driver
Growing Investments and Clinical Trial Expansion
- The increasing flow of public and private investments into exosome-based research, coupled with a surge in clinical trials across Europe, is a significant driver for the growing adoption of exosome therapeutics
- For instance, in March 2024, Evox Therapeutics (U.K.) expanded its collaboration with Janssen to accelerate exosome-based drug delivery programs for rare diseases, reinforcing the clinical and commercial potential of the field
- As healthcare systems seek new solutions for cancer, neurological, and cardiovascular diseases, exosome therapeutics offer advantages such as natural targeting properties and lower immunogenic risks compared to cell-based therapies
- Furthermore, the strong presence of academic centers and research clusters across Germany, France, and the U.K. is boosting discovery and innovation in exosome therapeutics, supported by EU funding programs and national grants
- The ability to attract major pharmaceutical collaborations, licensing deals, and government support is propelling the European ecosystem toward clinical maturity and commercial readiness. This reinforces exosome therapeutics as a high-growth segment of advanced biologics
- The rising focus on precision medicine and patient-specific treatment strategies across Europe is further strengthening the demand for exosome-based therapies, providing a favorable outlook for sustained market growth
Restraint/Challenge
Manufacturing Complexity and Regulatory Compliance Hurdle
- Concerns surrounding the complex manufacturing processes of exosome therapeutics, including isolation, scalability, and standardization, pose a significant challenge to broader market adoption. Ensuring reproducibility and quality remains a major hurdle
- For instance, regulatory authorities in Europe have highlighted the need for harmonized guidelines to ensure consistent quality control and safety of exosome-derived products, which can delay approvals and clinical progression
- Addressing these challenges through robust manufacturing technologies, standardized isolation protocols, and GMP-certified facilities is crucial for ensuring regulatory acceptance and market entry. Companies such as ExoPharm emphasize scalable platforms to overcome these barriers
- In addition, the relatively high production cost of exosome therapeutics compared to conventional biologics can limit accessibility, particularly in countries with tighter healthcare budgets or less established reimbursement frameworks
- While research advances are gradually improving scalability and cost-effectiveness, the perception of exosome therapeutics as premium biologics may still hinder widespread adoption among healthcare providers and payers
- Overcoming these challenges through regulatory harmonization, manufacturing innovation, and international collaborations will be vital for the sustained growth and commercialization of exosome therapeutics in Europe
Europe Exosome Therapeutic Market Scope
The market is segmented on the basis of type, source, therapy, transporting capacity, application, route of administration, end user, and distribution channel.
- By Type
On the basis of type, the exosome therapeutics market is segmented into natural exosomes and hybrid exosomes. The natural exosomes segment dominated the market with the largest revenue share in 2024, driven by their intrinsic biological origin, high biocompatibility, and minimal immunogenicity. Natural exosomes are widely preferred in clinical and preclinical research for delivering therapeutic molecules safely and effectively. Their ability to carry proteins, RNAs, and lipids to target cells has positioned them as a reliable vehicle in regenerative medicine and oncology. European biotech companies increasingly leverage natural exosomes for precision therapies due to regulatory familiarity and established safety profiles. Hospitals and research institutes prefer natural exosomes for translational studies and early-stage therapeutic applications. The growing number of clinical trials using natural exosomes reinforces their dominance across oncology, neurology, and metabolic disorder applications.
The hybrid exosomes segment is expected to witness the fastest growth during the forecast period, propelled by their engineered properties, higher payload capacity, and enhanced targeting ability. Hybrid exosomes, created by combining natural vesicles with synthetic materials, are increasingly adopted in gene therapy and immunotherapy pipelines to improve delivery efficiency. European biotech startups are exploring hybrid exosomes to overcome biological limitations of natural vesicles, such as short circulation time and lower stability. The flexibility to modify surface ligands and encapsulate diverse therapeutics makes hybrid exosomes highly attractive for next-generation precision medicine. Ongoing research collaborations across Germany, France, and the U.K. are accelerating hybrid exosome development, targeting both oncology and neurological disorders. As exosome engineering technologies mature, hybrid exosomes are expected to become commercially viable, driving high adoption rates.
- By Source
On the basis of source, the exosome therapeutics market is segmented into dendritic cells, mesenchymal stem cells (MSCs), blood, milk, body fluids, saliva, urine, and others. The mesenchymal stem cells (MSCs) segment dominated the market in 2024, owing to their abundant availability, strong exosome secretion profile, and immunomodulatory properties. MSC-derived exosomes are widely used in regenerative therapies, oncology, and cardiovascular applications. European research centers and hospitals extensively utilize MSC exosomes in preclinical and clinical trials due to consistent quality and well-established isolation protocols. MSC exosomes demonstrate enhanced tissue repair capabilities and can be engineered to carry therapeutic nucleic acids and proteins. Their scalability and reproducibility have made them a preferred source for commercial exosome therapeutics. Regulatory familiarity with MSC-derived products in Europe further supports their dominant market position.
The blood-derived exosomes segment is expected to witness the fastest growth during forecast period, driven by the rising use of plasma and serum exosomes in diagnostics, liquid biopsy, and drug delivery applications. Blood-derived exosomes are easily accessible and are being extensively investigated as biomarkers for cancer, cardiovascular, and metabolic disorders. European biotech companies are capitalizing on blood exosomes for non-invasive monitoring and targeted therapies. Technological advancements in isolation techniques have increased the yield and purity of blood exosomes, making them suitable for both clinical and research applications. Increasing collaborations between hospitals and biotech firms are accelerating the adoption of blood-derived exosomes. Rising interest in personalized medicine and early disease detection is fueling the rapid growth of this segment.
- By Therapy
On the basis of therapy, the exosome therapeutics market is segmented into immunotherapy, gene therapy, and chemotherapy. The oncology-focused immunotherapy segment dominated in 2024, driven by the increasing demand for targeted, cell-free treatments that enhance immune response while reducing systemic toxicity. Exosome-based immunotherapies are applied to stimulate anti-tumor activity and improve checkpoint inhibitor efficacy. European clinical trials in Germany, France, and the U.K. heavily invest in exosome-mediated immunotherapy platforms. The precision delivery capabilities of exosomes enhance antigen presentation and immune cell activation. Hospitals and research institutes are adopting immunotherapy exosomes due to their promising safety profile and therapeutic potential. Increasing government and EU funding for immuno-oncology accelerates clinical translation, reinforcing the dominance of this segment.
The gene therapy segment is expected to witness the fastest growth during forecast period, driven by the increasing application of exosomes as vectors for RNA and DNA therapeutics. Exosome-mediated gene therapy offers advantages such as lower immunogenicity and efficient delivery across biological barriers. European biotech firms, including those in the U.K. and Switzerland, are actively developing exosome-based RNA delivery systems targeting rare genetic disorders and cancer. Gene therapy applications benefit from ongoing technological innovations, enabling exosomes to carry CRISPR/Cas9 or siRNA payloads effectively. Rising collaborations between pharmaceutical companies and research institutes are accelerating clinical translation of gene therapies using exosomes. Regulatory support for advanced biologics further fuels the adoption of exosome-mediated gene therapy.
- By Transporting Capacity
On the basis of transporting capacity, the exosome therapeutics market is segmented into bio macromolecules and small molecules. The bio macromolecules segment dominated in 2024, owing to the ability of exosomes to carry proteins, nucleic acids, and complex biomolecules for therapeutic applications. Exosomes serve as natural carriers for RNA, DNA, and signaling proteins, enabling targeted delivery without eliciting significant immune reactions. European academic institutions and hospitals prefer exosome-mediated macromolecule transport for regenerative medicine, cancer therapy, and neurological disorders. High therapeutic efficacy, safety, and biocompatibility drive the preference for bio macromolecule exosome applications. Increasing R&D investments in complex biologics and precision medicine further support this segment’s market leadership. Scalable production platforms in Europe enhance availability for preclinical and clinical applications.
The small molecules segment is expected to witness the fastest growth during forecast period, driven by increasing research on drug-loaded exosomes for improved pharmacokinetics and targeted delivery. Small molecule therapeutics encapsulated in exosomes show enhanced bioavailability and reduced off-target effects. European biotech firms are developing exosome-based delivery systems for chemotherapy, anti-inflammatory agents, and metabolic drugs. Advances in encapsulation and loading techniques allow exosomes to deliver a variety of small molecule payloads efficiently. The adoption of small molecule exosome therapeutics is rising due to demand for minimally invasive and highly targeted therapies. Government funding and clinical trial activity in Europe are further boosting growth in this segment.
- By Application
On the basis of application, the exosome therapeutics market is segmented into oncology, neurology, metabolic disorders, cardiac disorders, blood disorders, inflammatory disorders, gynecology disorders, organ transplantation, and others. The oncology segment dominated in 2024 with a market share of 45.1%, driven by the urgent need for targeted cancer therapies with reduced side effects. Exosome therapeutics are applied for drug delivery, biomarker identification, and immunomodulation in oncology. European hospitals and research institutes are extensively using exosomes for both preclinical and clinical oncology studies. High cancer prevalence in Germany, France, and the U.K. boosts adoption. Exosome-based delivery enhances therapeutic efficacy while reducing systemic toxicity. Increasing partnerships between pharmaceutical companies and research institutions accelerate oncology-focused exosome development.
The neurology segment is expected to witness the fastest growth during forecast period, fueled by the ability of exosomes to cross the blood–brain barrier and deliver neuroprotective molecules. European research centers are exploring exosomes for Alzheimer’s, Parkinson’s, and stroke therapies. Exosome-mediated delivery enables precise targeting and sustained release of therapeutic molecules in the CNS. Rising prevalence of neurodegenerative disorders in aging European populations is driving demand. Clinical trials and academic research are accelerating the translation of exosome therapies in neurology. Government support for neurodegenerative research further propels segment growth.
- By Route of Administration
On the basis of route of administration, the exosome therapeutics market is segmented into oral and parenteral. The parenteral segment dominated in 2024, owing to higher bioavailability and the ability to deliver exosomes systemically or locally with precise dosing. Intravenous and subcutaneous administration is widely preferred for oncology, neurology, and immunotherapy applications. European hospitals and research institutes rely on parenteral routes for consistent therapeutic outcomes. Parenteral delivery allows controlled targeting to specific tissues or organs. The established clinical protocols in Europe favor parenteral administration for regulatory compliance. Advancements in formulation and delivery techniques enhance the safety and efficacy of parenteral exosome therapeutics.
The oral segment is expected to witness the fastest growth during forecast period, driven by research on bioengineered exosomes capable of surviving gastrointestinal conditions for systemic or localized delivery. Oral exosome therapeutics offer advantages in patient compliance and non-invasive administration. European biotech startups are developing protective coatings and encapsulation methods for oral exosome delivery. Rising interest in consumer-friendly therapeutic options and metabolic disorder treatments boosts adoption. Ongoing preclinical studies demonstrate feasibility and therapeutic potential. Regulatory encouragement for innovative delivery methods supports growth of oral exosome therapeutics.
- By End User
On the basis of end user, the exosome therapeutics market is segmented into hospitals, diagnostic centers, and research & academic institutes. The hospitals segment dominated in 2024, driven by direct clinical applications of exosome therapeutics in oncology, regenerative medicine, and immunotherapy. Hospitals provide access to patient populations for clinical trials and early-stage treatments. European hospitals in Germany, France, and the U.K. are increasingly integrating exosome-based therapies into clinical practice. Strong collaborations with biotech firms enhance therapeutic adoption. Hospitals are also equipped to monitor outcomes and manage complex biologics safely. Regulatory compliance and established treatment protocols reinforce hospital dominance.
The research and academic institutes segment is expected to witness the fastest growth during forecast period, fueled by expanding R&D activity in exosome therapeutics. Academic institutions across Europe are investing in novel exosome isolation, engineering, and delivery techniques. These institutes facilitate preclinical studies, proof-of-concept validation, and early-stage therapeutic development. Growing grants and EU funding programs support exosome research. Collaboration between academia and industry accelerates translation of research into clinical pipelines. The increasing focus on precision medicine and biotechnology education enhances segment growth.
- By Distribution Channel
On the basis of distribution channel, the exosome therapeutics market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated in 2024, as exosome therapeutics are primarily administered in clinical settings and require strict handling, storage, and administration protocols. Hospital pharmacies ensure adherence to GMP standards and provide controlled access for advanced biologics. European hospitals leverage pharmacy networks to deliver personalized exosome therapies safely to patients. Hospital pharmacies also support clinical trial supply chains and inventory management. Regulatory compliance and trained personnel reinforce this segment’s dominance.
The online pharmacy segment is expected to witness the fastest growth during forecast period, driven by increasing digitalization, telemedicine adoption, and the potential for direct-to-patient exosome therapeutic access in certain preclinical or companion diagnostic contexts. European biotech firms are exploring secure, regulated online distribution for research-use exosomes. Growth is supported by rising e-health infrastructure, patient convenience, and home-based administration trends. Secure cold-chain logistics enable broader online distribution. Government frameworks for telemedicine and remote healthcare facilitate growth. Online channels are expected to complement hospital distribution, especially for follow-up therapies and non-invasive applications.
Europe Exosome Therapeutic Market Regional Analysis
- Germany dominated the exosome therapeutics market with the largest revenue share of 41.8% in 2024, supported by strong R&D infrastructure, favorable regulatory frameworks for advanced therapies, and significant funding programs from both the EU and national government, with German biotech clusters leading in translational research and commercialization efforts
- Research institutions, hospitals, and biotech companies in the country are actively involved in developing and commercializing exosome-based therapies for oncology, neurology, and regenerative medicine, enhancing the adoption of these therapeutics across clinical settings
- This widespread adoption is further supported by a favorable regulatory framework for advanced therapies, high clinical trial activity, and collaborations between academic centers and pharmaceutical companies, establishing Germany as a key hub for exosome therapeutics in Europe
The Germany Exosome Therapeutics Market Insight
The Germany exosome therapeutics market dominated the Europe market with the largest revenue share in 2024, driven by well-established biotechnology clusters, extensive clinical trials, and early adoption of advanced biologics. Hospitals and research institutions actively explore exosome-based therapies for oncology, neurology, and regenerative medicine. Strong government support, EU funding programs, and collaborations between academia and pharmaceutical companies enhance clinical translation and commercialization. Germany’s emphasis on quality standards, innovation, and translational research strengthens its market leadership. Furthermore, the presence of leading biotech startups and established pharmaceutical firms accelerates R&D pipelines and adoption of exosome therapeutics.
France Exosome Therapeutics Market Insight
The France exosome therapeutics market is expected to witness the fastest growth in the Europe market during the forecast period, driven by increasing R&D investments, supportive government policies, and a rapidly expanding biotechnology sector. French hospitals and research centers are focusing on regenerative medicine, oncology, and neurological applications, while collaborations between biotech startups and pharmaceutical companies accelerate development and commercialization. Favorable regulatory frameworks and EU-backed funding initiatives provide a conducive environment for clinical translation. Rising prevalence of chronic and degenerative diseases further fuels demand. Innovation hubs in Paris, Lyon, and Marseille are accelerating the adoption of exosome therapeutics across hospitals and research institutes.
U.K. Exosome Therapeutics Market Insight
The U.K. exosome therapeutics market is projected to grow at a notable CAGR, driven by robust clinical research activity and investments in precision medicine. Academic institutions and biotech companies collaborate extensively to advance exosome-based therapies, particularly in oncology and immunotherapy. Government-backed funding initiatives and favorable regulatory frameworks support innovation and early-stage commercialization. The U.K.’s well-developed biotech ecosystem, strong infrastructure, and focus on translational medicine contribute to increasing adoption.
Italy Exosome Therapeutics Market Insight
The Italy exosome therapeutics market is witnessing steady growth in the market, driven by expanding clinical research, collaborations between hospitals and biotech firms, and increased focus on regenerative medicine. Italian healthcare institutions actively engage in preclinical and early-stage clinical studies for exosome-based therapies, particularly in oncology and cardiovascular applications. Government initiatives supporting biotechnology facilitate innovation. Rising prevalence of chronic diseases and demand for advanced therapeutics boost market adoption.
Europe Exosome Therapeutic Market Share
The Europe exosome therapeutic industry is primarily led by well-established companies, including:
- EXO Biologics (Belgium)
- Exosomics Inc. (Italy)
- EVerzom (France)
- AEGLE Therapeutics (U.S.)
- VivaZome Therapeutics Pty Ltd (Australia)
- ILIAS Biologics Inc. (U.S.)
- SOTIO Biotech (Czech Republic)
- ProQR Therapeutics (Netherlands)
- AvenCell Therapeutics, Inc. (Germany)
- Miltenyi Biotec (Germany)
- Amylon Therapeutics (Netherlands)
- Exosome Diagnostics (U.S.)
- Capricor Therapeutics, Inc. (U.S.)
- Aruna Bio (U.S.)
- Evox Therapeutics (U.K.)
- Exosome Therapeutics (U.S.)
- Exosome Plus (U.S.)
What are the Recent Developments in Europe Exosome Therapeutic Market?
- In April 2024, Memel Biotech announced a Memorandum of Understanding (MoU) with THERABEST Korea to research and develop exosome and other innovative cell therapies. This collaboration aims to advance therapeutic applications in the exosome field
- In March 2024, HansaBioMed Life Sciences, in collaboration with the Finnish Red Cross Blood Service, introduced purified extracellular vesicles from human platelets. This advancement enhances the standardization and purity of extracellular vesicles, supporting research in diagnostics and therapeutic applications
- In January 2024, INNOAESTHETICS introduced INNO-EXOMA EXO-SKIN, Europe's first fully synthetic exosome product. This innovative treatment targets skin regeneration, alopecia, and other dermatological concerns, marking a significant advancement in regenerative aesthetics
- In January 2024, Aegle Therapeutics commenced a Phase 1/2 clinical trial for AGLE-102, a therapeutic composite of extracellular vesicles, including exosomes, for treating severe second-degree burns. The first patient dosed achieved 99% epithelialization within seven days, demonstrating promising results.
- In July 2023, Researchers in Europe have developed novel methods for enhancing the stability and targeting efficiency of exosome-based drug delivery systems. These advancements aim to improve the therapeutic efficacy of exosome-based treatments for various diseases, including cancer and autoimmune disorders
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.