Europe Heart Valve Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
3.38 Billion
USD
8.88 Billion
2024
2032
USD
3.38 Billion
USD
8.88 Billion
2024
2032
| 2025 –2032 | |
| USD 3.38 Billion | |
| USD 8.88 Billion | |
|
|
|
|
Europe Heart Valve Devices Market Size
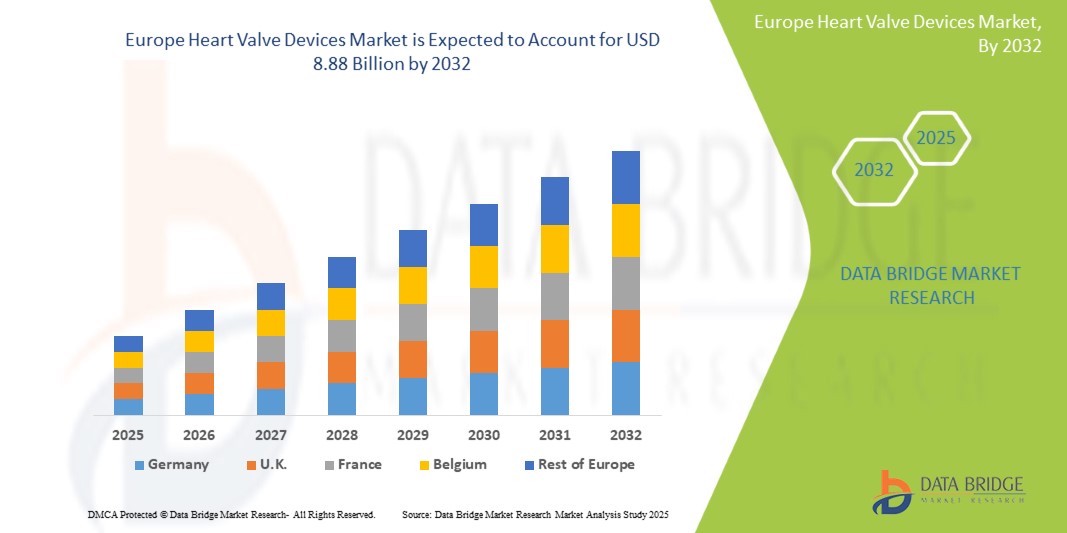
- The Europe heart valve devices market size was valued at USD 3.38 billion in 2024 and is expected to reach USD 8.88 billion by 2032, at a CAGR of 12.8% during the forecast period
- The market growth is primarily driven by the increasing prevalence of cardiovascular diseases, aging population, and advancements in minimally invasive valve replacement and repair technologies across the region
- Moreover, rising awareness about heart health, improved healthcare infrastructure, and growing adoption of transcatheter procedures are fueling demand for heart valve devices in Europe. These factors collectively contribute to the steady expansion of the heart valve devices market throughout the forecast period.
Europe Heart Valve Devices Market Analysis
- Heart valve devices, including mechanical and bioprosthetic valves used for valve replacement and repair, are essential in treating valvular heart diseases, playing a critical role in improving patient outcomes and quality of life across European healthcare systems
- The increasing prevalence of cardiovascular disorders, rising geriatric population, and growing preference for minimally invasive transcatheter valve procedures are key factors driving demand for heart valve devices in the region
- Germany dominated the Europe heart valve devices market with the largest revenue share of 22.9% in 2024, supported by advanced healthcare infrastructure, high adoption rates of cutting-edge technologies, and strong reimbursement policies
- U.K. is expected to be the fastest-growing country within Europe heart valve devices market during the forecast period due to increasing healthcare investments, growing patient awareness, and expanding access to innovative valve therapies
- The transcatheter valve segment dominated the Europe heart valve devices market with a share of 52.8% in 2024, propelled by its minimally invasive nature, shorter recovery times, and expanding indication approvals
Report Scope and Europe Heart Valve Devices Market Segmentation
|
Attributes |
Europe Heart Valve Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Europe Heart Valve Devices Market Trends
Advancements in Minimally Invasive and Transcatheter Technologies
- A key and rapidly growing trend in the Europe heart valve devices market is the widespread adoption of minimally invasive and transcatheter valve replacement and repair procedures, which reduce patient recovery time and surgical risks compared to traditional open-heart surgeries
- For instance, Edwards Lifesciences’ Sapien valve and Medtronic’s CoreValve systems have revolutionized aortic valve replacement with transcatheter approaches, gaining broad clinical acceptance across European cardiac centers. Similarly, Abbott’s MitraClip device offers a minimally invasive solution for mitral valve repair
- Innovations such as improved valve durability, repositionable valve systems, and enhanced delivery catheters are driving increased procedural success rates and expanding patient eligibility, including those previously considered inoperable. These advances support the growing preference among physicians and patients for less invasive treatment options
- The integration of advanced imaging and navigation technologies with valve devices facilitates precise implantation, improving outcomes and reducing complications. Leading companies such as Boston Scientific and JenaValve Technologies are focusing on developing such integrated systems
- This shift toward minimally invasive therapies is reshaping treatment paradigms in Europe, with greater adoption expected as reimbursement policies evolve and clinical guidelines increasingly endorse transcatheter approaches for broader patient populations
- Demand for heart valve devices that offer innovative, less invasive solutions is rising sharply due to improved patient quality of life and shorter hospital stays, creating substantial growth opportunities for manufacturers across Europe
Europe Heart Valve Devices Market Dynamics
Driver
Increasing Prevalence of Cardiovascular Diseases and Aging Population
- The rising incidence of valvular heart diseases driven by aging demographics and lifestyle-related cardiovascular risk factors is a major growth driver for the Europe heart valve devices market
- For instance, in 2024, Germany reported a significant increase in transcatheter aortic valve replacement (TAVR) procedures due to expanded indication approvals and growing awareness among clinicians and patients. This trend is mirrored in other major countries such as France and the U.K.
- The expanding elderly population increases the demand for effective and safer heart valve therapies, fueling investments in research, device innovation, and healthcare infrastructure to support advanced valve procedures
- Improvements in diagnostic capabilities, including echocardiography and cardiac MRI, are enabling earlier detection of valvular conditions, further driving device adoption
- Government initiatives and favorable reimbursement frameworks in countries such as Italy and Spain also enhance accessibility to innovative heart valve therapies, boosting market growth
Restraint/Challenge
High Procedure Costs and Complex Regulatory Environment
- The relatively high costs associated with heart valve devices and related surgical procedures remain a significant barrier, limiting accessibility, especially in countries with constrained healthcare budgets
- For instance, despite clinical benefits, some Eastern European countries show slower adoption rates due to funding limitations and lack of reimbursement parity compared to Western Europe
- The stringent regulatory environment in Europe, including rigorous CE marking processes and compliance with the Medical Device Regulation (MDR), poses challenges for manufacturers, potentially delaying product launches and increasing development costs
- Variability in clinical guidelines and physician preferences across countries can also slow the uniform adoption of novel valve technologies
- Addressing cost barriers through health economic studies demonstrating long-term benefits, expanding reimbursement coverage, and streamlining regulatory pathways will be essential for sustained market expansion
Europe Heart Valve Devices Market Scope
The market is segmented on the basis of product type, treatment, end user, and distribution channel.
- By Type
On the basis of product type, the Europe heart valve devices market is segmented into mechanical heart valves, biological heart valves, and transcatheter valves. The transcatheter valves segment dominated the market with the largest revenue share of 52.8% in 2024, largely due to its minimally invasive procedure, which offers patients reduced recovery times, lower risk of complications, and suitability for high-risk or elderly patients who may not be candidates for open-heart surgery. Expanding clinical approvals and increased physician preference further fuel this dominance.
The biological heart valves segment is expected to witness the fastest growth during the forecast period. This is driven by continuous improvements in valve durability, reduced risk of thrombosis compared to mechanical valves, and increasing patient preference, particularly among the aging population who benefit from avoiding lifelong anticoagulation therapy. Mechanical heart valves, while historically important for their long-term durability especially in younger patients, show steady but slower growth compared to other segments, as newer valve technologies gain traction.
- By Treatment
On the basis of treatment, the Europe heart valve devices market is segmented into open surgery and minimally invasive surgery (MIS). The minimally invasive surgery segment dominated the market in 2024, driven by the growing adoption of transcatheter aortic valve replacement (TAVR), transcatheter mitral valve repair, and robotic-assisted valve surgeries. These approaches offer advantages such as smaller incisions, less trauma, shorter hospital stays, faster recovery, and fewer complications, making them highly preferred by both patients and clinicians.
The open surgery segment is expected to witness fastest growth during forecast period, particularly for complex cases and younger patients requiring durable mechanical valves, but it is anticipated to experience slower growth as MIS techniques become more widespread and accessible.
- By End User
On the basis of end user, the Europe heart valve devices market is categorized into hospitals & clinics, ambulatory surgical centers, cardiac centers, research centers, and others. Hospitals & clinics dominated the market with the largest revenue share in 2024, supported by their established infrastructure, experienced surgical teams, and capability to manage complex cardiac procedures. These facilities handle the majority of heart valve device implantations across Europe.
Cardiac centers is expected to witness the fastest CAGR from 2025 to 2032, due to their specialized focus on cardiovascular diseases, investment in cutting-edge technologies, and increasing procedure volumes. These centers offer targeted, high-quality care, attracting more patients and referrals.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into direct tender and third-party distributors. The direct tender segment dominated the market in 2024, driven by large healthcare providers, government health agencies, and public hospitals procuring heart valve devices directly from manufacturers. This channel offers advantages such as cost savings through bulk purchasing, assured supply, and direct technical support.
The third-party distributors segment is anticipated to be the fastest growing during the forecast period. This growth is attributed to increasing penetration in smaller hospitals, private clinics, and emerging European markets, where distributors facilitate broader geographic reach, quicker deliveries, and diversified product offerings. Partnerships and collaborations between manufacturers and distributors are strengthening this channel, improving accessibility to innovative heart valve devices and driving overall market expansion.
Europe Heart Valve Devices Market Regional Analysis
- Germany dominated the Europe heart valve devices market with the largest revenue share of 22.9% in 2024, supported by advanced healthcare infrastructure, high adoption rates of cutting-edge technologies, and strong reimbursement policies
- Patients and healthcare providers in Germany prioritize minimally invasive and transcatheter valve procedures due to their benefits in reducing recovery time and improving clinical outcomes, further boosting market demand
- The widespread adoption of cutting-edge heart valve technologies is supported by significant investments in cardiovascular care, growing awareness of valvular heart diseases, and a robust network of specialized cardiac centers across the country, establishing Germany as a key market leader in Europe
The Germany Heart Valve Devices Market Insight
The Germany heart valve devices market dominates the Europe market with the largest revenue share in 2024, supported by its well-established cardiovascular care network and strong governmental support for healthcare innovation. The country’s focus on research and development has fostered the adoption of cutting-edge heart valve devices such as transcatheter aortic valve replacement (TAVR) and robotic-assisted surgeries. Moreover, high healthcare spending and reimbursement coverage make these advanced therapies accessible to a broad patient base. Increasing public awareness about valvular heart diseases and improved diagnostic capabilities further boost the demand for heart valve devices.
U.K. Heart Valve Devices Market Insight
The U.K. heart valve devices market is expected to grow at a robust CAGR during the forecast period, driven by rising investments in cardiac healthcare and a growing geriatric population vulnerable to valvular diseases. The implementation of enhanced screening and early detection programs is increasing diagnosis rates, leading to higher procedural volumes. In addition, the U.K.’s well-developed healthcare system and emphasis on minimally invasive treatment options, including TAVR, are significant growth factors. Patient preference for faster recovery and less invasive therapies is encouraging widespread adoption of advanced valve devices.
France Heart Valve Devices Market Insight
The France heart valve devices market is witnessing steady growth due to expanding cardiac care facilities and increasing adoption of transcatheter and minimally invasive valve repair technologies. Progressive healthcare policies and strong public-private collaborations are facilitating faster penetration of innovative devices in the market. Patient demand for less invasive treatment options is rising, supported by favorable clinical outcomes and improved quality of life post-procedure, which drives device adoption in both public and private healthcare settings.
Italy Heart Valve Devices Market Insight
The Italy heart valve devices market is showing gradual yet consistent growth, aided by improvements in healthcare infrastructure and increasing awareness of cardiovascular conditions. The growing use of minimally invasive surgical techniques, supported by expanding regional cardiac centers, is driving demand for advanced valve therapies. Government initiatives focused on cardiovascular health and funding for innovative technologies are also contributing to market expansion, especially in major urban centers.
Europe Heart Valve Devices Market Share
The Europe Heart Valve Devices industry is primarily led by well-established companies, including:
- Edwards Lifesciences Corporation (U.S.)
- Medtronic (Ireland)
- Abbott (U.S.)
- Boston Scientific Corporation (U.S.)
- JenaValve Technology, Inc. (Germany)
- CryoLife, Inc. (U.S.)
- LivaNova PLC (U.K.)
- Terumo Corporation (Japan)
- MicroPort Scientific Corporation (China)
- Sorin Group (Italy)
- Meril Life Sciences Pvt. Ltd. (India)
- Braile Biomédica (Brazil)
- Lepu Medical Technology Co., Ltd. (China)
- Cardiovalve Ltd. (Israel)
- Colibri Heart Valve GmbH (Germany)
- Pi-Cardia Ltd. (Israel)
- InnovHeart SAS (France)
- PulseCath BV (Netherlands)
- Reliance Medical Products (U.K.)
- HeartWare International, Inc. (U.S.)
What are the Recent Developments in Europe Heart Valve Devices Market?
- In July 2025, Boston Scientific announced it will discontinue sales of its Acurate neo2 and Acurate Prime TAVR systems in Europe, following a failed clinical study and stringent regulatory barriers. As a result, Edwards Lifesciences has seen an uptick in its market position, raising its 2025 sales guidance and reinforcing its dominance in transcatheter therapies
- In May 2025, Medtronic has received a CE Mark in Europe expanding the indication for its Evolut Pro+ and FX transcatheter aortic valve systems to be used in “redo TAVR” procedures. These systems can now be implanted within previously failed transcatheter heart valves—regardless of the original manufacturer providing a critical treatment option for high‑risk patients facing valve failure
- In January 2025, Medtronic received CE Mark approval for the Harmony TPV system, a transcatheter pulmonary valve designed for congenital heart disease patients with right ventricular outflow tract (RVOT) issues. Already used in over 2,200 patients, this minimally invasive alternative broadens treatment access for European patients requiring pulmonary valve repair
- In May 2024, Edwards Lifesciences introduced the SAPIEN 3 Ultra RESILIA transcatheter aortic valve in Europe following CE Mark approval. Leveraging anti‑calcification RESILIA tissue and dry packaging, this valve offers enhanced durability and ease of use. Real‑world data highlight its favorable hemodynamic performance and low rates of paravalvular leak compared to prior model
- In December 2024, Meril Life Sciences unveiled its next‑generation transcatheter heart valve, the Myval Octapro THV, in Europe. Designed to improve operator control and implant predictability, the device features a comprehensive size matrix from traditional to extra‑large dimensions to accommodate varied patient anatomies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.