Europe Lysosomal Storage Disorder Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
2.92 Billion
USD
5.68 Billion
2024
2032
USD
2.92 Billion
USD
5.68 Billion
2024
2032
| 2025 –2032 | |
| USD 2.92 Billion | |
| USD 5.68 Billion | |
|
|
|
Lysosomal Storage Disorder Drugs Market Analysis
The Europe lysosomal storage disorder drugs market encompasses the commercial sector for pharmaceuticals developed to diagnose, treat, and manage various lysosomal storage disorders, which are rare genetic conditions typically characterized by the accumulation of undigested substances within lysosomes due to enzyme deficiencies. This market includes a range of therapeutic products, such as enzyme replacement therapies, substrate reduction therapies, and gene therapies, aimed at addressing the diverse clinical manifestations of LSDs, such as Gaucher disease, Fabry disease, and Pompe disease. With increasing awareness, advancements in research and technology, and a growing number of pipeline therapies, this market is poised for significant expansion. In addition, rising healthcare expenditure and initiatives to improve access to rare disease treatments further drive market growth, presenting both opportunities and challenges for pharmaceutical companies and healthcare providers.
Lysosomal Storage Disorder Drugs Market Size
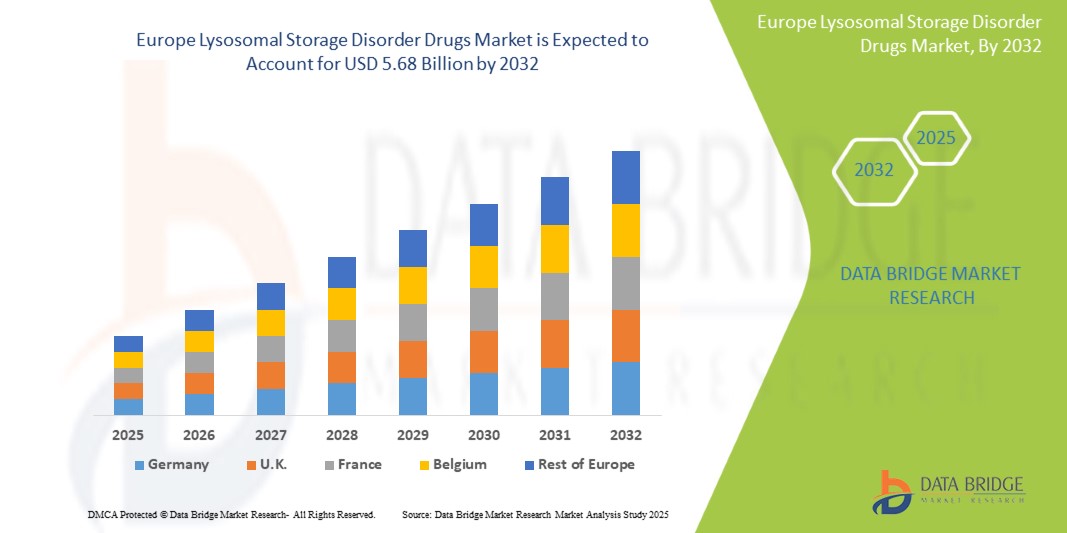
Europe lysosomal storage disorder drugs market size was valued at USD 2.92 billion in 2024 and is projected to reach USD 5.68 billion by 2032, growing with a CAGR of 8.7% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework.
Lysosomal Storage Disorder Drugs Market Trends
“Biotechnology Advancements for Lysosomal Storage Disorders Treatments”
Advancements in biotechnology have played a pivotal role in transforming the treatment landscape for Lysosomal Storage Disorders (LSDs), providing patients with more effective and tailored treatment options. Enzyme Replacement Therapy (ERT) has revolutionized the treatment of Lysosomal Storage Disorders (LSDs) by directly addressing the enzyme deficiencies that cause these conditions. In LSD patients, the lack of specific enzymes leads to the accumulation of toxic substances in cells, damaging organs and tissues. ERT works by administering synthetic enzymes to compensate for the missing ones, improving metabolic functions and alleviating symptoms. Over the years, ERT has advanced significantly, with new, more effective formulations and delivery methods that improve enzyme absorption and reduce side effects. These innovations have resulted in better clinical outcomes, this trend slowed disease progression, reduced organ damage, and enhanced patient quality of life.
Report Scope and Lysosomal Storage Disorder Drugs Market Segmentation
|
Attributes |
Lysosomal Storage Disorder Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Germany, U.K., Italy, France, Spain, Russia, Switzerland, Turkey, Belgium, Netherlands, Denmark, Sweden, Poland, Norway, Finland, and rest of Europe |
|
Key Market Players |
Sanofi (France), BioMarin (U.S.), Pfizer Inc. (U.S.), Amicus Therapeutics, Inc. (U.S.), Takeda Pharmaceutical Company Limited (Japan), Ultragenyx Pharmaceutical Inc. (U.S.), Orchard Therapeutics plc (U.K.), Spur Therapeutics (U.K.), Sangamo Therapeutics (U.S.), CHIESI Farmaceutici S.p.A. (Italy), REGENXBIO INC. (U.S.), and JCR Pharmaceuticals Co., Ltd. (Japan) among others |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Lysosomal Storage Disorder Drugs Market Definition
Lysosomal storage disorder drugs refer to a category of pharmaceuticals specifically developed to treat Lysosomal Storage Disorders (LSDs), which are a group of rare genetic conditions caused by deficiencies in specific enzymes responsible for breaking down complex molecules within lysosomes. These drugs aim to address the underlying metabolic abnormalities associated with LSDs by either replacing the missing enzymes (enzyme replacement therapy), by enhancing the body's ability to produce the enzymes, or by modifying the substrates that accumulate due to enzyme deficiency (substrate reduction therapy). By improving enzymatic function or reducing the toxic build-up of substrates, these treatments can alleviate symptoms, slow disease progression, and ultimately improve the quality of life for individuals affected by these challenging conditions.
Lysosomal Storage Disorder Drugs Market Dynamics
Drivers
- Emerging Personalized Medicine for Lysosomal Storage Disorders
Emerging personalized medicine is transforming the landscape of treatment for Lysosomal Storage Disorders (LSDs) by offering therapies specifically tailored to the individual genetic profiles of patients. LSDs are caused by genetic mutations that affect enzyme function, and these mutations can vary widely between patients. Personalized treatments involve analyzing a patient’s unique genetic makeup to develop a more targeted approach, optimizing the efficacy of therapies such as enzyme replacement or gene therapy. This precision allows for better matching of treatments with the patient's specific needs, minimizing side effects and enhancing therapeutic outcomes. As advancements in genetic testing and technology progress, healthcare providers are able to identify the most suitable interventions, potentially improving response rates and overall treatment success. The shift toward personalized medicine is accelerating the development of customized LSD treatments, which promise to revolutionize disease management. With these innovations, the global lysosomal storage disorders drug market is witnessing increased demand for more effective, individualized treatment options. Personalized medicine is thus a key driver of market growth, as it boosts treatment effectiveness and enhances patient outcomes, fostering greater confidence in therapeutic options.
For instance,
In February 2023, according to the article published by NCBI, Emerging personalized medicine uses an individual’s genetic profile to guide decisions related to the prevention, diagnosis, and treatment of diseases. This approach enables more precise and effective therapies for Lysosomal Storage Disorders (LSDs), tailoring treatments to specific genetic mutations. As personalized medicine becomes more prevalent, it drives the demand for targeted LSD therapies, fueling growth in the global lysosomal storage disorders drug market.
- Increased Government Funding for Research into Rare Disease Treatments
Government funding and grants for research into rare disease treatments are crucial for advancing the development of therapies for conditions such as Lysosomal Storage Disorders (LSDs). Rare diseases, often lacking commercial viability due to small patient populations, pose significant challenges in drug development. To address this, governments worldwide are increasingly allocating funds to incentivize research in these underserved areas. Financial support in the form of grants, subsidies, and tax credits helps pharmaceutical companies and research institutions overcome the high costs and risks associated with developing treatments for rare diseases. Furthermore, governments often fast-track regulatory processes for these treatments, recognizing the urgent need for solutions. By reducing the financial burden on researchers and companies, these funding mechanisms enable the exploration of innovative therapies, such as enzyme replacement and gene therapies that would otherwise face significant barriers to development.
For instance,
In August 2023, according to the article published by National Organization for Rare Diseases, The National Organization for Rare Disorders announced over USD 100,000 in grant funding for rare disease research, highlighting increased government support for advancing treatments. These funds enable researchers to explore innovative therapies for rare diseases like Lysosomal Storage Disorders (LSDs). Such financial backing accelerates drug development, driving growth in the global LSD drug market by fostering new treatment options.
Opportunities
- Increasing Number of Pipeline Drugs
The increasing number of drugs in the pipeline for Lysosomal Storage Disorders (LSDs) represents a significant opportunity for the global lysosomal storage disorders drugs market, potentially leading to improved treatment options and enhanced patient outcomes. As researchers and pharmaceutical companies enhance their understanding of these disorders, they are developing a diverse array of novel therapies, including gene therapies, substrate reduction therapies, and small molecule drugs. This expanded pipeline reflects a growing recognition of the unmet medical needs within the LSD community and promises to offer patients a wider variety of treatment modalities tailored to their specific conditions. The introduction of innovative therapies can enhance patient adherence to treatment, reduce disease burden, and ultimately improve the quality of life for individuals living with these disorders.
For instance,
In June 2024, Ultragenyx announced the Planning to file for accelerated approval of UX111 for the treatment of Sanfilippo Syndrome Type A (MPS IIIA). UX111 is a novel in vivo gene therapy in Phase 1/2/3 development for Sanfilippo syndrome type A (MPS IIIA), a rare fatal lysosomal storage disease with no approved treatment that primarily affects the central nervous system.
- Growing Emphasis on Early Diagnosis
The growing emphasis on early diagnosis of Lysosomal Storage Disorders (LSDs) presents a significant opportunity for the global lysosomal storage disorders drugs market by facilitating timely interventions that can greatly enhance patient outcomes. As healthcare systems place greater emphasis on early detection through improved screening programs, genetic testing, and advancements in diagnostic technologies, more patients are likely to be diagnosed at an earlier stage of their diseases. This early intervention allows for better management of symptoms but increases the potential efficacy of existing and emerging therapies. The result of this shift is a broader patient base that requires and can benefit from treatment options, ultimately driving demand within the lysosomal storage disorders drug market.
For instance,
In February 2023, according to an article, ‘Lysosomal storage disorders: from biology to the clinic with reference to India’, early diagnosis is the most critical part of the management of LSDs as it provides the opportunity for therapeutic intervention, precise genetic counselling, prenatal diagnosis and a better outcome for the patient.
Restraints/Challenges
- Lack of Awareness Among Healthcare Professionals and Patients
The lack of awareness among healthcare professionals and patients about Lysosomal Storage Disorders (LSDs) presents a significant barrier to early diagnosis, treatment, and effective management. Many healthcare providers, especially in regions with limited exposure to rare diseases, often fail to recognize the symptoms of LSDs, which are diverse and overlap with other more common conditions. This lack of knowledge leads to delayed diagnoses, misdiagnosis, and ineffective treatment, exacerbating the severity of the disease. For patients, especially those in resource-poor or underserved areas, the lack of awareness prevents early intervention, leaving them unaware of potential therapies. The complexity and rarity of LSDs further complicate the situation, as patients and healthcare professionals may not fully understand the importance of early treatment or the availability of specialized care options. Fewer patients are diagnosed and treated in time, leading to suboptimal clinical outcomes. This lack of awareness limits the demand for LSD-specific treatments and hinders the overall growth of the global lysosomal storage disorders drugs market.
For instance,
In January 2024, according to the article published by Wiley, there’s a significant diagnostic delay of around 15 years from symptom onset to diagnosis is common in adult LSD cases due to overlapping clinical phenotypes and varying severity. This delay highlights the lack of awareness among healthcare professionals, particularly those treating adult patients. Such restraints hinder early detection and timely treatment, restricting the growth of the global lysosomal storage disorders drugs market.
- Prolonged Drug Approval Procedures
Lengthy drug approval processes present a significant restraint for the Europe lysosomal storage disorder drugs market, slowing the introduction of much-needed therapies. The process for obtaining regulatory approval for new drugs, particularly for rare diseases like LSDs, involves numerous clinical trials, extensive documentation, and rigorous assessments by regulatory bodies such as the FDA and EMA. These approval timelines are often extended due to the need for a thorough evaluation of safety, efficacy, and potential long-term effects. In the case of LSDs, the rarity and complexity of these diseases add another layer of challenge, as there is a limited patient population for clinical trials, making it difficult to generate robust data. The need for specialized treatments and the lack of established standards for these rare diseases complicates the approval process. Consequently, drug developers face prolonged waiting periods, delays in bringing new treatments to market, and additional costs, which can be discouraging for pharmaceutical companies. As a result, the lengthy approval processes slow the availability of innovative therapies for patients, reducing market growth and hindering the accessibility of effective treatments for LSDs. This regulatory delay ultimately acts as a major restraint for the global lysosomal storage disorders drugs market.
For instance,
In August 2024, according to the article published by Drugs.com, The research, development, and approval process for drugs typically takes 12 to 15 years. This extended timeline delays the introduction of new therapies and increases costs for pharmaceutical companies. As a result, the lengthy drug approval process restrains the growth of the global LSD drug market by limiting the speed of treatment availability.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Lysosomal Storage Disorder Drugs Market Scope
The market is segmented on the basis of by type of disorder, type, drugs, route of administration, age group, gender, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type of Disorder
- Gaucher Disease
- Type 1
- Type 3
- Type 2
- Fabry Disease
- Pompe Disease
- Infantile-Onset Pompe
- Late-Onset Pompe
- Mucopolysaccharidosis (MPS)
- MPS I
- MPS II
- MPS IV
- MPS VI
- MPS III
- Niemann-Pick Disease
- Type C
- Type B
- Type A
- Krabbe Disease
- Others
Type
- Enzyme Replacement Therapy (ERT)
- Substrate Reduction Therapy (SRT)
- Chaperone Therapy
- Others
Drugs
- Imiglucerase
- Agalsidase Beta
- Idursulfase
- Alglucosidase Alpha
- Velaglucerase
- Taliglucerase Alfa
- Laronidase
- Agalsidase Alpha
- Galsulfase
- Avalglucosidase Alfa
- Others
Route of Administration
- Intravenous (IV)
- Subcutaneous (SC)
- Oral
- Others
Age Group
- Pediatric
- Adults
- Geriatric
Gender
- Male
- Female
Distribution Channel
- Hospital Pharmacies
- Drugs Stores and Retail Pharmacies
- Online Pharmacies
Lysosomal Storage Disorder Drugs Market Regional Analysis
The market is analyzed and market size insights and trends are provided by type of disorder, type, drugs, route of administration, age group, gender, and distribution channel as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, U.K., Italy, France, Spain, Russia, Switzerland, Turkey, Belgium, Netherlands, Denmark, Sweden, Poland, Norway, Finland, and rest of Europe.
Germany is expected to dominate and the fastest growing country in Europe lysosomal storage disorder drugs market due to increasing healthcare infrastructure, rising prevalence of lysosomal storage diseases, and growing awareness about advanced therapies.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Europe brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Lysosomal Storage Disorder Drugs Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Europe presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Lysosomal Storage Disorder Drugs Market Leaders Operating in the Market Are:
- Sanofi (France)
- BioMarin (U.S.)
- Pfizer Inc. (U.S.)
- Amicus Therapeutics, Inc. (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Orchard Therapeutics plc (U.K.)
- Spur Therapeutics (U.K.)
- Sangamo Therapeutics (U.S.)
- CHIESI Farmaceutici S.p.A. (Italy)
- REGENXBIO INC. (U.S.)
- JCR Pharmaceuticals Co., Ltd. (Japan)
Latest Developments in Lysosomal Storage Disorder Drugs Market
- In October 2024, Amicus Therapeutics announced a settlement regarding its drug Galafold (migalastat), which treats Fabry disease. The settlement resolves ongoing patent disputes and confirms the continuation of the drug’s marketing without litigation interference. This agreement with multiple parties aims to provide stability for Galafold’s availability and development while protecting intellectual property rights
- In June 2024, Amicus Therapeutics was honored with the Prix Galien UK Award for its innovative treatment, Pombiliti (miglustat), for the management of Fabry disease. The award recognizes excellence in pharmaceutical innovation and emphasizes the impact of Pombiliti in improving the lives of patients with rare genetic conditions. This accolade highlights Amicus’ leadership in rare disease therapies
- In April 2024, Forge Biologics announced it would present nine times at the ASGCT 27th Annual Meeting in May 2024, including a late-breaking oral presentation and three technical sessions. Presentations will cover process development, molecular advancements, and clinical updates, including a significant clinical result for FBX-101 in Krabbe disease
- In November 2023, Chiesi Group has been reaccredited as a Great Place to Work-Certified organization across multiple regions, including Italy, Australia, the US, and others. With an 85% response rate from employees, Chiesi achieved an 83% overall satisfaction rate, reflecting its commitment to fostering a positive, inclusive, and collaborative work environment focused on employee well-being and growth
- In January 2024, Denali Therapeutics Inc., a biopharmaceutical company developing therapies to cross the blood-brain barrier for treating neurodegenerative and lysosomal storage diseases, announced progress and milestones for 2024. CEO Ryan Watts, Ph.D., highlighted these developments during a corporate presentation at the 42nd Annual J.P. Morgan Healthcare Conference on January 9th
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET
1.4 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 SECONDARY SOURCES
2.1 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PORTER’S FIVE FORCES
4.2 PIPELINE ANALYSIS
5 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: REGULATIONS
5.1 REGULATORY AUTHORITIES IN EUROPE
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 BIOTECHNOLOGY ADVANCEMENTS FOR LYSOSOMAL STORAGE DISORDERS TREATMENTS
6.1.2 EMERGING PERSONALIZED MEDICINE FOR LYSOSOMAL STORAGE DISORDERS
6.1.3 INCREASED GOVERNMENT FUNDING FOR RESEARCH INTO RARE DISEASE TREATMENTS
6.1.4 COLLABORATIONS AND PARTNERSHIPS BETWEEN PHARMACEUTICAL COMPANIES AND RESEARCH INSTITUTIONS
6.2 RESTRAINTS
6.2.1 LACK OF AWARENESS AMONG HEALTHCARE PROFESSIONALS AND PATIENTS
6.2.2 PROLONGED DRUG APPROVAL PROCEDURES
6.3 OPPORTUNITIES
6.3.1 INCREASING NUMBER OF PIPELINE DRUGS
6.3.2 GROWING EMPHASIS ON EARLY DIAGNOSIS
6.3.3 ADVANCEMENTS IN GENE THERAPY FOR THE TREATMENT OF LYSOSOMAL STORAGE DISORDERS
6.4 CHALLENGES
6.4.1 SIGNIFICANT EXPENSES RELATED TO THE TREATMENT OF THE DISEASE
6.4.2 NARROW PATIENT BASE SUFFERING FROM LYSOSOMAL STORAGE DISORDERS
7 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER
7.1 OVERVIEW
7.2 GAUCHER DISEASE
7.2.1 TYPE 1
7.2.2 TYPE 3
7.2.3 TYPE 2
7.3 FABRY DISEASE
7.4 POMPE DISEASE
7.4.1 INFANTILE-ONSET POMPE
7.4.2 LATE-ONSET POMPE
7.5 MUCOPOLYSACCHARIDOSIS (MPS)
7.5.1 MPS I
7.5.2 MPS II
7.5.3 MPS IV
7.5.4 MPS VI
7.5.5 MPS III
7.6 NIEMANN-PICK DISEASE
7.6.1 TYPE C
7.6.2 TYPE B
7.6.3 TYPE A
7.7 KRABBE DISEASE
7.8 OTHERS
8 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS
8.1 OVERVIEW
8.2 IMIGLUCERASE
8.3 AGALSIDASE BETA
8.4 IDURSULFASE
8.5 ALGLUCOSIDASE ALPHA
8.6 VELAGLUCERASE
8.7 TALIGLUCERASE ALFA
8.8 LARONIDASE
8.9 AGALSIDASE ALPHA
8.1 GALSULFASE
8.11 AVALGLUCOSIDASE ALFA
8.12 OTHERS
9 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE
9.1 OVERVIEW
9.2 ENZYME REPLACEMENT THERAPY (ERT)
9.3 SUBSTRATE REDUCTION THERAPY (SRT)
9.4 CHAPERONE THERAPY
9.5 OTHERS
10 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER
10.1 OVERVIEW
10.2 MALE
10.3 FEMALE
11 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP
11.1 OVERVIEW
11.2 PEDIATRIC
11.3 ADULT
11.4 GERIATRIC
12 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 HOSPITAL PHARMACIES
12.3 DRUGS STORES AND RETAIL PHARMACIES
12.4 ONLINE PHARMACIES
13 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 INTRAVENOUS (IV)
13.3 SUBCUTANEOUS (SC)
13.4 ORAL
13.5 OTHERS
14 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION
14.1 EUROPE
14.1.1 GERMANY
14.1.2 UNITED KINGDOM
14.1.3 FRANCE
14.1.4 ITALY
14.1.5 SPAIN
14.1.6 RUSSIA
14.1.7 SWITZERLAND
14.1.8 TURKEY
14.1.9 BELGIUM
14.1.10 NETHERLANDS
14.1.11 DENMARK
14.1.12 SWEDEN
14.1.13 POLAND
14.1.14 NORWAY
14.1.15 FINLAND
14.1.16 REST OF EUROPE
15 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 SWOT ANALYSIS
17 COMPANY PROFILES
17.1 SANOFI
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 BIOMARIN
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENT
17.3 PFIZER INC.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENT
17.4 TAKEDA PHARMACEUTICAL COMPANY LIMITED
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.5 AMICUS THERAPEUTIC, INC.
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENT
17.6 CHIESI FARMACEUTICI S.P.A.
17.6.1 COMPANY SNAPSHOT
17.6.2 PRODUCT PORTFOLIO
17.6.3 RECENT DEVELOPMENT
17.7 DENALI THERAPEUTICS
17.7.1 COMPANY SNAPSHOT
17.7.2 REVENUE ANALYSIS
17.7.3 PIPELINE PRODUCT PORTFOLIO
17.7.4 RECENT DEVELOPMENT
17.8 FORGE BIOLOGICS
17.8.1 COMPANY SNAPSHOT
17.8.2 PIPELINE PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENT
17.9 JCR PHARMACEUTICALS CO., LTD.
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PINELINE PRODUCT PORTFOLIO
17.9.4 PRODUCT PORTFOLIO
17.9.5 RECENT DEVELOPMENT
17.1 ORCHARD THERAPEUTICS PLC
17.10.1 COMPANY SNAPSHOT
17.10.2 REVENUE ANALYSIS
17.10.3 PRODUCT PORTFOLIO
17.10.4 RECENT DEVELOPMENT
17.11 PROTALIX BIOTHERAPEUTICS
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PIPELINE PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENT
17.12 REGENXBIO INC.
17.12.1 COMPANY SNAPSHOT
17.12.2 REVENUE ANALYSIS
17.12.3 PINELINE PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENT
17.13 SANGAMO THERAPEUTICS
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 SPUR THERAPEUTICS
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENT
17.15 ULTRAGENYX PHARMACEUTICAL INC.
17.15.1 COMPANY SNAPSHOT
17.15.2 REVENUE ANALYSIS
17.15.3 PRODUCT PORTFOLIO
17.15.4 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
List of Table
TABLE 1 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 2 EUROPE GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 3 EUROPE GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 4 EUROPE FABRY DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 5 EUROPE POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 6 EUROPE POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 7 EUROPE MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 8 EUROPE MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 9 EUROPE NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 10 EUROPE NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 11 EUROPE KRABBE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 12 EUROPE OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 13 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 14 EUROPE IMIGLUCERASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 15 EUROPE AGALSIDASE BETA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 16 EUROPE IDURSULFASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 17 EUROPE ALGLUCOSIDASE ALPHA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 18 EUROPE VELAGLUCERASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 19 EUROPE TALIGLUCERASE ALFA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 20 EUROPE LARONIDASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 21 EUROPE AGALSIDASE ALPHA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 22 EUROPE GALSULFASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 23 EUROPE AVALGLUCOSIDASE ALFA IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 24 EUROPE OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 25 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 26 EUROPE ENZYME REPLACEMENT THERAPY (ERT) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 27 EUROPE SUBSTRATE REDUCTION THERAPY (SRT) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 28 EUROPE CHAPERONE THERAPY IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 29 EUROPE OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 30 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 31 EUROPE MALE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 32 EUROPE FEMALE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 33 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 34 EUROPE PEDIATRIC IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 35 EUROPE ADULT IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 36 EUROPE GERIATRIC IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 37 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 38 EUROPE HOSPITAL PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 39 EUROPE DRUGS STORES AND RETAIL PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 40 EUROPE ONLINE PHARMACIES IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 41 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 42 EUROPE INTRAVENOUS (IV) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032(USD MILLION)
TABLE 43 EUROPE SUBCUTANEOUS (SC) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 44 EUROPE ORAL IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 45 EUROPE OTHERS IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY REGION, 2018-2032 (USD MILLION)
TABLE 46 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY COUNTRY, 2018-2032 (USD MILLION)
TABLE 47 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 48 EUROPE GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 49 EUROPE POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 50 EUROPE MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 51 EUROPE NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 52 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 53 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 54 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 55 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 56 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 57 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 58 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 59 GERMANY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 60 GERMANY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 61 GERMANY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 62 GERMANY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 63 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 64 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 65 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 66 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 67 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 68 GERMANY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 69 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 70 UNITED KINGDOM GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 71 UNITED KINGDOM POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 72 UNITED KINGDOM MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 73 UNITED KINGDOM NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 74 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 75 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 76 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 77 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 78 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 79 UNITED KINGDOM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 80 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 81 FRANCE GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 82 FRANCE POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 83 FRANCE MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 84 FRANCE NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 85 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 86 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 87 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 88 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 89 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 90 FRANCE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 91 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 92 ITALY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 93 ITALY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 94 ITALY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 95 ITALY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 96 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 97 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 98 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 99 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 100 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 101 ITALY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 102 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 103 SPAIN GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 104 SPAIN POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 105 SPAIN MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 106 SPAIN NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 107 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 108 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 109 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 110 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 111 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 112 SPAIN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 113 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 114 RUSSIA GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 115 RUSSIA POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 116 RUSSIA MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 117 RUSSIA NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 118 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 119 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 120 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 121 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 122 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 123 RUSSIA LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 124 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 125 SWITZERLAND GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 126 SWITZERLAND POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 127 SWITZERLAND MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 128 SWITZERLAND NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 129 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 130 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 131 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 132 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 133 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 134 SWITZERLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 135 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 136 TURKEY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 137 TURKEY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 138 TURKEY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 139 TURKEY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 140 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 141 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 142 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 143 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 144 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 145 TURKEY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 146 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 147 BELGIUM GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 148 BELGIUM POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 149 BELGIUM MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 150 BELGIUM NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 151 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 152 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 153 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 154 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 155 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 156 BELGIUM LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 157 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 158 NETHERLANDS GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 159 NETHERLANDS POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 160 NETHERLANDS MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 161 NETHERLANDS NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 162 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 163 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 164 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 165 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 166 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 167 NETHERLANDS LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 168 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 169 DENMARK GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 170 DENMARK POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 171 DENMARK MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 172 DENMARK NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 173 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 174 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 175 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 176 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 177 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 178 DENMARK LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 179 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 180 SWEDEN GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 181 SWEDEN POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 182 SWEDEN MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 183 SWEDEN NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 184 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 185 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 186 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 187 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 188 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 189 SWEDEN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 190 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 191 POLAND GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 192 POLAND POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 193 POLAND MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 194 POLAND NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 195 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 196 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 197 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 198 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 199 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 200 POLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 201 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 202 NORWAY GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 203 NORWAY POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 204 NORWAY MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 205 NORWAY NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 206 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 207 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 208 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 209 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 210 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 211 NORWAY LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 212 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
TABLE 213 FINLAND GAUCHER DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 214 FINLAND POMPE DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 215 FINLAND MUCOPOLYSACCHARIDOSIS (MPS) IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 216 FINLAND NIEMANN-PICK DISEASE IN LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 217 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE, 2018-2032 (USD MILLION)
TABLE 218 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DRUGS, 2018-2032 (USD MILLION)
TABLE 219 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2018-2032 (USD MILLION)
TABLE 220 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY AGE GROUP, 2018-2032 (USD MILLION)
TABLE 221 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY GENDER, 2018-2032 (USD MILLION)
TABLE 222 FINLAND LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD MILLION)
TABLE 223 REST OF EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER, 2018-2032 (USD MILLION)
List of Figure
FIGURE 1 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SEGMENTATION
FIGURE 2 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DROC ANALYSIS
FIGURE 4 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: MULTIVARIATE MODELLING
FIGURE 7 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SEGMENTATION
FIGURE 10 EXECUTIVE SUMMARY
FIGURE 11 SEVEN SEGMENTS COMPRISE THE EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET, BY TYPE OF DISORDER
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 BIOTECHNOLOGY ADVANCEMENTS FOR LYSOSOMAL STORAGE DISORDER TREATMENTS IS EXPECTED TO DRIVE THE EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET IN THE FORECAST PERIOD
FIGURE 14 GAUCHER DISEASE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET IN 2025 & 2032
FIGURE 15 PESTEL ANALYSIS
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET
FIGURE 17 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, 2024
FIGURE 18 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, 2025-2032 (USD MILLION)
FIGURE 19 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, CAGR (2025-2032)
FIGURE 20 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE OF DISORDER, LIFELINE CURVE
FIGURE 21 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, 2024
FIGURE 22 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, 2025-2032 (USD MILLION)
FIGURE 23 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, CAGR (2025-2032)
FIGURE 24 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DRUGS, LIFELINE CURVE
FIGURE 25 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, 2024
FIGURE 26 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, 2025-2032 (USD MILLION)
FIGURE 27 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, CAGR (2025-2032)
FIGURE 28 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 29 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, 2024
FIGURE 30 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, 2025-2032 (USD MILLION)
FIGURE 31 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, CAGR (2025-2032)
FIGURE 32 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY GENDER, LIFELINE CURVE
FIGURE 33 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, 2024
FIGURE 34 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, 2025-2032 (USD MILLION)
FIGURE 35 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, CAGR (2025-2032)
FIGURE 36 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 37 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 38 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD MILLION)
FIGURE 39 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032)
FIGURE 40 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 41 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, 2024
FIGURE 42 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, 2025-2032 (USD MILLION)
FIGURE 43 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2025-2032)
FIGURE 44 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 45 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: SNAPSHOT 2024
FIGURE 46 EUROPE LYSOSOMAL STORAGE DISORDER DRUGS MARKET: COMPANY SHARE 2024 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.