Europe Migraine Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
1,398.94 Million
USD
3,640.75 Million
2022
2030
USD
1,398.94 Million
USD
3,640.75 Million
2022
2030
| 2023 –2030 | |
| USD 1,398.94 Million | |
| USD 3,640.75 Million | |
|
|
|
Europe Migraine Treatment Market Analysis and Size
Migraine is a common and debilitating brain illness. Headache accounts for 4.4 percent of all primary care visits, nearly 5% of all medical hospital admissions, and more than 20% of neurology outpatient consultations. Migraine affects approximately 20% of people at some point in their lives, according to epidemiological research, 4.5% of Western Europe's population suffers from headaches at least 15 days per month. The vast majority of medications on the market now are approved to treat the acute version of the illness with generic triptans serving as the primary therapy. With the advent of CGRP-based medicines and the acceptance of novel medication classes such as ditans, gepants and the reformulation of triptans for both chronic and episodic migraine, the industry is observing a shift in terms of significant R&D.
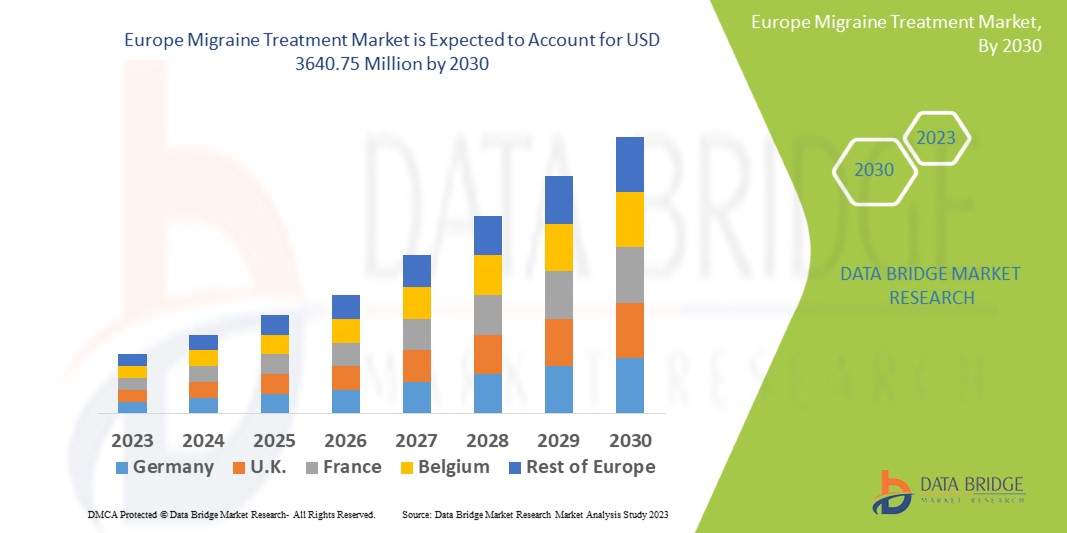
Data Bridge Market Research analyses that the migraine treatment market which is USD 1398.94 million in 2022, is expected to reach USD 3640.75 million by 2030, at a CAGR of 12.7% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Europe Migraine Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Types (Episodic, Migraine with Aura, Chronic and Others), Treatment (Acute/Abortive Treatment, Preventive/Prophylactic Treatment, Non-Pharmacological Therapies and Devices), Route of Administration (Oral, Parenteral, Nasal Sprays and Others), Product Type (Prescription and Over the Counter), Drug Type (Branded and Generic), End User (Hospitals, Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies and Others) |
|
Countries Covered |
Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe |
|
Market Players Covered |
Pfizer Inc. (U.S.), Eli Lilly and Company (U.S.), Amgen Inc. (U.S.), GSK plc. (U.K.), Novartis AG (Switzerland), Bayer AG (Germany), Abbott (U.S.), Allodynic Therapeutics, LLC (U.S.), AOBiome (U.S.), AstraZeneca (U.K.), Aurobindo Pharma USA (U.S.), Bausch Health Companies Inc. (Canada), Biohaven Pharmaceuticals (U.S.), Boehringer Ingelheim International GmbH (Germany), Catalent, Inc (U.S.), Dr. Reddy’s Laboratories Ltd. (India), Impel Pharmaceuticals Inc. (U.S.), H. Lundbeck A/S (Denmark), Merck & Co., Inc (U.S.) |
|
Market Opportunities |
|
Market Definition
Migraine is a debilitating neurological disease characterized by recurrent severe throbbing head pain attacks lasting more than three days. Migraine affects approximately 15% of the population over their lifetime with women (18%) being more affected than men (8%). It has been dubbed the "seventh disabler" due to its significant impact on patients' quality of life (QOL).
Migraine Treatment Market Dynamics
Drivers
• Increase in migraine prevalence and diagnosis
An enormous increase in migraine cases is a key factor fuelling market expansion. Additionally, the industry is gaining from better migraine treatment reimbursement rules and the increased need for precision medications. The market is expanding due to rising demand for electrical nerve stimulators used to treat migraines and the anticipated approval of new classes of late-stage pipeline medications with higher clinical efficacy, such as the introduction of calcitonin gene-related peptide (CGRP) monoclonal antibodies.
- Significant progress is being made in the preventative treatment of chronic migraine
The important developments should force to revaluate how to treat this illness and encourage to keep an eye out for new developments in clinical science, and reminds that only a coordinated clinical and scientific management approach will increase the identification of chronic migraines. The distinction between phenotypic and biological indicators sheds light on areas of clinical governance that have gone unnoticed. It is up to ensure that cutting-edge and evolving therapy options, including OBT-A and anti-CGRP monoclonal antibodies, are fully utilized within a larger preventative culture to significantly reduce the societal, monetary and individual costs of this fatal illness.
Opportunities
- Growing awareness regarding migraine
The key factors influencing the growth of the Europe migraine treatment market in the near future are the rising prevalence of migraines and the variety of available treatments. Many organizations, such as the American Headache and Migraine Association, are working to increase public awareness of migraine by creating programmes and offering patient support. These efforts aim to increase understanding of the condition and associated headache conditions. Many vendors will invest in migraine medications' research and development (R&D) due to the growing incidence of migraines and unmet needs in the Europe migraine treatment market. As a result, during the forecast period of 2023 to 2030, the market for migraine treatment is anticipated to expand.
Restraints/Challenges
- Side effects associated with migraine treatment
The drop in clinical visits and procedural treatment of migraines due to social distance norms, limits of current migraine medicines and alternative therapies such as homoeopathy are anticipated to hamper the market growth in the forecast period of 2023 to 2030.
This migraine treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the migraine treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Migraine Treatment Market
The neurologic manifestations of COVID-19 suggest that the virus may have entered the nervous system through the olfactory groove or the bloodstream. There is compelling evidence that COVID-19 mortality is linked to cardiac and pulmonary diseases. Furthermore, migraine increases the burden of vascular diseases, putting migraine sufferers at a higher risk of poor COVID-19 outcomes. Acute COVID-19 symptoms such as fever, sleep disturbance, and dehydration may precipitate a migraine attack. COVID-19 headaches were described as pulsating, pressing, or stabbing in quality, were mostly bilateral, lasted longer, were resistant to analgesia and were more common in men. This makes migraine sufferers particularly vulnerable to the pandemic's chronic and indirect effects, such as low mood and post-viral fatigue. After the pandemic rising R&D spending resulting in massive pipeline products, as well as an increase in the prevalence of migraines with high unmet needs are all contributing to the growth of the migraine treatment market.
Recent Development
- In 2022, Biohaven Pharmaceutical Holding Company Ltd. and Pfizer Inc., Rimegepant,introduced a calcitonin gene-related peptide (CGRP) receptor antagonist. The 75 mg dose of Rimegepant (available as an orally dissolving tablet) is suggested for marketing authorization for the acute treatment of migraine with or without aura in adults and the preventative treatment for episodic migraine in adults who experience at least four migraine attacks per month.
Europe Migraine Treatment Market Scope
The migraine treatment market is segmented on the basis of types, treatment, route of administration, product, distribution channel, drug type and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Types
- Episodic
- Migraine with Aura
- Chronic
- Others
Treatment
- Acute/Abortive Treatment
- Preventive/prophylactic Treatment
- Non-Pharmacological Therapies and Devices
Route of Administration
- Oral
- Parenteral
Product
- Prescription
- Over the Counter
Drug Type
- Branded
- Generic
End-User
- Hospitals
- Clinics
- Homecare
- Others
Distribution Channel
- Hospital-Based Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Migraine Treatment Market Regional Analysis/Insights
The migraine treatment market is analyzed and market size insights and trends are provided by country, type, treatment, route of administration, product, distribution channel, drug type and end-user as referenced above.
The countries covered in the migraine treatment market report are Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe.
Germany is dominating the Europe migraine treatment market due to the vast number of businesses present in the market and the strong emphasis placed on innovation and API production. Additionally, pharmaceutical industry investment has increased significantly in recent years.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Europe brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The migraine treatment market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for migraine treatment market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the migraine treatment market. The data is available for historic period 2011-2021.
Competitive Landscape and Migraine Treatment Market Share Analysis
The migraine treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Europe presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to migraine treatment market.
Some of the major players operating in the migraine treatment market are:
- Pfizer Inc. (U.S.)
- Eli Lilly and Company (U.S.)
- Amgen Inc. (U.S.)
- GSK plc. (U.K.)
- Novartis AG (Switzerland)
- Bayer AG (Germany)
- Abbott (U.S.)
- Allodynic Therapeutics, LLC (U.S.)
- AOBiome (U.S.)
- AstraZeneca (U.K.)
- Aurobindo Pharma USA (U.S.)
- Bausch Health Companies Inc. (Canada)
- Biohaven Pharmaceuticals (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- Catalent, Inc (U.S.)
- Dr. Reddy’s Laboratories Ltd. (India)
- Impel Pharmaceuticals Inc. (U.S.)
- H. Lundbeck A/S (Denmark)
- Merck & Co., Inc (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE MIGRAINE TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE EUROPE MIGRAINE TREATMENT SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 EUROPE MIGRAINE TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 INDUSTRY INSIGHTS
5.1 PATENT ANALYSIS
5.1.1 PATENT LANDSCAPE
5.1.2 USPTO NUMBER
5.1.3 PATENT EXPIRY
5.1.4 EPIO NUMBER
5.1.5 PATENT STRENGTH AND QUALITY
5.1.6 PATENT CLAIMS
5.1.7 PATENT CITATIONS
5.1.8 PATENT LITIGATION AND LICENSING
5.1.9 FILE OF PATENT
5.1.10 PATENT RECEIVED CONTRIES
5.1.11 TECHNOLOGY BACKGROUND
5.2 DRUG TREATMENT RATE BY MATURED MARKETS
5.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.4 PATIENT FLOW DIAGRAM
5.5 KEY PRICING STRATEGIES
5.6 KEY PATIENT ENROLLMENT STRATEGIES
5.7 INTERVIEWS WITH SPECIALIST
5.8 OTHER KOL SNAPSHOTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATIENT TREATMENT SUCCESS RATES
7 MERGERS AND ACQUISITION
7.1 LICENSING
7.2 COMMERCIALIZATION AGREEMENTS
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 EUROPE CLINICAL TRIAL MARKET FOR MIGRAINE TREATMENT
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yest Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR MIGRAINE TREATMENT
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
10 MARKETED DRUG ANALYSIS
10.1 DRUG
10.1.1 BRAND NAME
10.1.2 GENERICS NAME
10.2 THERAPEUTIC INDIACTION
10.3 PHARACOLOGICAL CLASS OD THE DRUG
10.4 DRUG PRIMARY INDICATION
10.5 MARKET STATUS
10.6 MEDICATION TYPE
10.7 DRUG DOSAGES FORM
10.8 DOSAGES AVAILABILITY
10.9 PACKAGING TYPE
10.1 DRUG ROUTE OF ADMINISTRATION
10.11 DOSING FREQUENCY
10.12 DRUG INSIGHT
10.13 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
10.13.1 FORECAST MARKET OUTLOOK
10.13.2 CROSS COMPETITION
10.13.3 THERAPEUTIC PORTFOLIO
10.13.4 CURRENT DEVELOPMENT SCENARIO
11 MARKET ACCESS
11.1 10-YEAR MARKET FORECAST
11.2 CLINICAL TRIAL RECENT UPDATES
11.3 ANNUAL NEW FDA APPROVED DRUGS
11.4 DRUGS MANUFACTURER AND DEALS
11.5 MAJOR DRUG UPTAKE
11.6 CURRENT TREATMENT PRACTICES
11.7 IMPACT OF UPCOMING THERAPY
12 R & D ANALYSIS
12.1 COMPARATIVE ANALYSIS
12.2 DRUG DEVELOPMENTAL LANDSCAPE
12.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
12.4 THERAPEUTIC ASSESSMENT
12.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
13 MARKET OVERVIEW
13.1 DRIVERS
13.2 RESTRAINTS
13.3 OPPORTUNITIES
13.4 CHALLENGES
14 EUROPE MIGRAINE TREATMENT MARKET, BY TYPES OF MIGRAINE
14.1 OVERVIEW
14.2 MIGRAINE WITH AURA (COMPLICATED MIGRAINE)
14.3 MIGRAINE WITH AURA (COMMON MIGRAINE)
14.4 MIGRAINE WITHOUT HEAD PAIN
14.5 RETINAL MIGRAINE (OCULAR MIGRAINE)
14.6 HEMIPLEGIC MIGRAINE
14.7 RETINAL MIGRAINE (OCULAR MIGRAINE)
14.8 MIGRAINE WITH BRAINSTEM AURA
14.9 STATUS MIGRAINOSUS
14.1 CHRONIC MIGRAINE
15 EUROPE MIGRAINE TREATMENT MARKET, BY TREATMENT
15.1 OVERVIEW
15.2 MEDICATIONS
15.2.1 ACUTE / ABORTIVE TREATMENT
15.2.1.1. NONSPECIFIC THERAPIES
15.2.1.1.1. NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
15.2.1.1.1.1 ASPIRIN
15.2.1.1.1.2 IBUPROFEN
15.2.1.1.1.3 ACETAMINOPHEN
15.2.1.1.1.4 OTHERS
15.2.1.1.2. OPIATES/OPOIDS
15.2.1.1.2.1 OXYCONTIN
15.2.1.1.2.2 VICODIN
15.2.1.1.2.3 PERCOCET
15.2.1.1.2.4 OTHERS
15.2.1.1.3. TRIPTANS
15.2.1.1.3.1 RIZATRIPTAN
15.2.1.1.3.2 SUMATRIPTAN
15.2.1.1.3.3 OTHERS
15.2.1.1.4. ERGOT ALKALOID
15.2.1.1.4.1 DIHYDROERGOTAMINES
15.2.1.1.4.2 ERGOTAMINE
15.2.1.1.4.3 LASMIDITAN
15.2.1.1.4.4 OTHERS
15.2.1.1.5. ANTI-NAUSEA
15.2.1.1.5.1 CHLORPROMAZINE
15.2.1.1.5.2 METOCLOPRAMIDE
15.2.1.1.5.3 PROCHLORPERAZINE
15.2.1.1.5.4 OTHERS
15.2.1.1.6. OTHERS
15.2.2 ADJUNCTIVE THERAPIES
15.2.2.1. ANTIEMETICS
15.2.2.2. SEDATIVES
15.2.2.3. OTHERS
15.2.3 OTHERS
15.2.3.1. INTRANASAL LIDOCAINE
15.2.3.2. STEROIDS
15.2.3.3. OTHERS
15.2.4 PREVENTIVE/ PROPHYLACTIC TREATMENT
15.2.5 BLOOD PRESSURE-LOWERING MEDICATIONS
15.2.5.1. BETA BLOCKERS
15.2.5.1.1. INDERAL (PROPRANAOLOL)
15.2.5.1.2. TIMOLOL
15.2.5.1.3. OTHERS
15.2.5.2. CALCIUM-CHANNEL BLOCKERS
15.2.5.2.1. VERAPAMIL (CALAN)
15.2.5.2.2. NIMODIPINE (NIMOTOP)
15.2.5.2.3. OTHERS
15.2.6 ANTICONVULSANTS
15.2.6.1. DEPAKOTE (DIVALPROEX SODIUM)
15.2.6.2. TOPAMAX (TOPIRAMATE)
15.2.6.3. QUDEXY XR (TOPIRAMATE)
15.2.6.4. TROKENDI (TOPIRAMATE)
15.2.6.5. OTHERS
15.2.7 ANGIOTENSIN BLOCKERS: ACE-IS/ARBS
15.2.7.1. CANDESARTAN
15.2.7.2. TELMISARTAN
15.2.7.3. OTHERS
15.2.8 NSAIDS
15.2.8.1. FENOPROFEN
15.2.8.2. KETOPROFEN
15.2.8.3. NAPROXEN
15.2.8.4. NAPROXEN SODIUM
15.2.8.5. OTHERS
15.2.9 TRIPTANS
15.2.9.1. FROVATRIPTAN
15.2.9.2. NARATRIPTAN
15.2.9.3. OTHERS
15.2.10 CALCITONIN GENE-RELATED PEPTIDE THERAPY
15.2.10.1. ERENUMAB-AOOE
15.2.10.2. FREMANEZUMAB-VFRM
15.2.10.3. GALCANEZUMAB
15.2.10.4. REMANEZUMAB
15.2.10.5. OTHERS
15.2.11 ANTIDEPRESSANTS
15.2.11.1. AMITRIPTYLINE
15.2.11.2. NORTRIPTYLINE
15.2.11.3. OTHERS
15.2.12 SELECTIVE SEROTONIN RECEPTOR AGONISTS
15.2.12.1. ALMOTRIPTAN MALATE
15.2.12.2. RELPAX (ELETRIPTAN)
15.2.12.3. FROVA (FROVATRIPTAN)
15.2.12.4. AMERGE (NARATRIPTAN)
15.2.12.5. MAXALT (RIZATRIPTAN)
15.2.12.6. IMITREX (SUMATRIPTAN)
15.2.12.7. ZOMIG (ZOLMITRIPTAN)
15.2.12.8. OTHERS
15.2.13 OTHERS
15.3 NON-PHARMACOLOGICAL THERAPIES
15.3.1 ACUPUNCTURE
15.3.2 MASSAGE
15.3.3 COGNITIVE BEHAVIOR THERAPY
15.3.4 OTHERS
15.4 DEVICES
15.4.1 SUPRAORBITAL STIMULATION
15.4.1.1. CEFALY DEVICE
15.4.1.2. RELIVION
15.4.2 VAGUS NERVE STIMULATION (VNS)
15.4.3 OCCIPITAL NERVE STIMULATION (ONS)
15.4.3.1. ANKERSTIM
15.4.3.2. REMOTE ELECTRICAL NEUROMODULATION (REN)
15.4.3.3. SINGLE-PULSE TRANSCRANIAL MAGNETIC STIMULATION (STMS)
15.4.3.3.1. SAVI DUAL
15.4.3.3.2. OTHERS
15.5 OTHERS
16 EUROPE MIGRAINE TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
16.1 OVERVIEW
16.2 ORAL
16.3 PARENTERAL
16.4 NASAL SPRAYS
16.5 OTHERS
17 EUROPE MIGRAINE TREATMENT MARKET, BY DRUY TYPE
17.1 OVERVIEW
17.2 BRANDED
17.2.1 ZOMIG
17.2.2 MAXALT
17.2.3 AXERT
17.2.4 FROVA
17.2.5 RELPAX
17.2.6 IMITREX
17.2.7 CAFERGOT
17.2.8 TYLENOL
17.2.9 CALPOL
17.2.10 ZAVZPRET
17.2.11 REYVOW
17.2.12 UBRELVY
17.2.13 TOSYMRA
17.2.14 MAXALT
17.2.15 VYEPTI
17.2.16 PANADOL
17.2.17 OTHERS
17.3 GENERIC
18 EUROPE MIGRAINE TREATMENT MARKET, BY MODE OF PURCHASE
18.1 OVERVIEW
18.2 PRESCRIPTION
18.3 OVER THE COUNTER
19 EUROPE MIGRAINE TREATMENT MARKET, BY GENDER
19.1 OVERVIEW
19.2 MALE
19.3 FEMALE
20 EUROPE MIGRAINE TREATMENT MARKET, BY END USER
20.1 OVERVIEW
20.2 HOSPITALS
20.3 BY TYPE
20.3.1.1. PUBLIC
20.3.1.2. PRIVATE
20.3.2 BY TIER
20.3.2.1. TIER 1
20.3.2.2. TIER 2
20.3.2.3. TIER 3
20.4 CLINICS
20.5 SPECIALITY CLINICS
20.6 HOMCARE
20.7 OTHERS
21 EUROPE MIGRAINE TREATMENT MARKET, BY DISTRIBUTION CHANNEL
21.1 OVERVIEW
21.2 DIRECT TENDERS
21.3 HOSPITAL PHARMACY
21.4 RETAIL PHARMACY
21.5 ONLINE PHARMACY
21.6 OTHERS
22 EUROPE MIGRAINE TREATMENT MARKET, SWOT AND DBMR ANALYSIS
23 EUROPE MIGRAINE TREATMENT MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: EUROPE
23.2 MERGERS & ACQUISITIONS
23.3 NEW PRODUCT DEVELOPMENT & APPROVALS
23.4 EXPANSIONS
23.5 REGULATORY CHANGES
23.6 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 EUROPE MIGRAINE TREATMENT MARKET, BY REGION
24.1 EUROPE MIGRAINE TREATMENT MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.2 EUROPE
24.2.1 GERMANY
24.2.2 U.K.
24.2.3 ITALY
24.2.4 FRANCE
24.2.5 SPAIN
24.2.6 RUSSIA
24.2.7 SWITZERLAND
24.2.8 TURKEY
24.2.9 BELGIUM
24.2.10 NETHERLANDS
24.2.11 DENMARK
24.2.12 SWEDEN
24.2.13 POLAND
24.2.14 NORWAY
24.2.15 FINLAND
24.2.16 REST OF EUROPE
24.3 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 EUROPE MIGRAINE TREATMENT MARKET, COMPANY PROFILE
25.1 PFIZER INC.
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPMENTS
25.2 ELI LILLY AND COMPANY
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPMENTS
25.3 AMGEN INC.
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPMENTS
25.4 GSK PLC
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPMENTS
25.5 NOVARTIS AG
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPMENTS
25.6 BAYER AG
25.6.1 COMPANY OVERVIEW
25.6.2 REVENUE ANALYSIS
25.6.3 GEOGRAPHIC PRESENCE
25.6.4 PRODUCT PORTFOLIO
25.6.5 RECENT DEVELOPMENTS
25.7 MEDTRONIC
25.7.1 COMPANY OVERVIEW
25.7.2 REVENUE ANALYSIS
25.7.3 GEOGRAPHIC PRESENCE
25.7.4 PRODUCT PORTFOLIO
25.7.5 RECENT DEVELOPMENTS
25.8 ABBVIE INC.
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPMENTS
25.9 NEUROLIEF INC.
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPMENTS
25.1 ASTRAZENECA
25.10.1 COMPANY OVERVIEW
25.10.2 REVENUE ANALYSIS
25.10.3 GEOGRAPHIC PRESENCE
25.10.4 PRODUCT PORTFOLIO
25.10.5 RECENT DEVELOPMENTS
25.11 AUROBINDO PHARMA
25.11.1 COMPANY OVERVIEW
25.11.2 REVENUE ANALYSIS
25.11.3 GEOGRAPHIC PRESENCE
25.11.4 PRODUCT PORTFOLIO
25.11.5 RECENT DEVELOPMENTS
25.12 BAUSCH HEALTH COMPANIES INC.
25.12.1 COMPANY OVERVIEW
25.12.2 REVENUE ANALYSIS
25.12.3 GEOGRAPHIC PRESENCE
25.12.4 PRODUCT PORTFOLIO
25.12.5 RECENT DEVELOPMENTS
25.13 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
25.13.1 COMPANY OVERVIEW
25.13.2 REVENUE ANALYSIS
25.13.3 GEOGRAPHIC PRESENCE
25.13.4 PRODUCT PORTFOLIO
25.13.5 RECENT DEVELOPMENTS
25.14 CATALENT, INC
25.14.1 COMPANY OVERVIEW
25.14.2 REVENUE ANALYSIS
25.14.3 GEOGRAPHIC PRESENCE
25.14.4 PRODUCT PORTFOLIO
25.14.5 RECENT DEVELOPMENTS
25.15 DR. REDDY’S LABORATORIES LTD.
25.15.1 COMPANY OVERVIEW
25.15.2 REVENUE ANALYSIS
25.15.3 GEOGRAPHIC PRESENCE
25.15.4 PRODUCT PORTFOLIO
25.15.5 RECENT DEVELOPMENTS
25.16 H. LUNDBECK A/S
25.16.1 COMPANY OVERVIEW
25.16.2 REVENUE ANALYSIS
25.16.3 GEOGRAPHIC PRESENCE
25.16.4 PRODUCT PORTFOLIO
25.16.5 RECENT DEVELOPMENTS
25.17 MERCK & CO., INC
25.17.1 COMPANY OVERVIEW
25.17.2 REVENUE ANALYSIS
25.17.3 GEOGRAPHIC PRESENCE
25.17.4 PRODUCT PORTFOLIO
25.17.5 RECENT DEVELOPMENTS
25.18 ELECTROCORE, INC.
25.18.1 COMPANY OVERVIEW
25.18.2 REVENUE ANALYSIS
25.18.3 GEOGRAPHIC PRESENCE
25.18.4 PRODUCT PORTFOLIO
25.18.5 RECENT DEVELOPMENTS
25.19 THERANICA BIO-ELECTRONICS LTD.
25.19.1 COMPANY OVERVIEW
25.19.2 REVENUE ANALYSIS
25.19.3 GEOGRAPHIC PRESENCE
25.19.4 PRODUCT PORTFOLIO
25.19.5 RECENT DEVELOPMENTS
25.2 ENEURA INC.
25.20.1 COMPANY OVERVIEW
25.20.2 REVENUE ANALYSIS
25.20.3 GEOGRAPHIC PRESENCE
25.20.4 PRODUCT PORTFOLIO
25.20.5 RECENT DEVELOPMENTS
25.21 CEFALY TECHNOLOGY
25.21.1 COMPANY OVERVIEW
25.21.2 REVENUE ANALYSIS
25.21.3 GEOGRAPHIC PRESENCE
25.21.4 PRODUCT PORTFOLIO
25.21.5 RECENT DEVELOPMENTS
25.22 TEVA PHARAMCEUTICAL INC,
25.22.1 COMPANY OVERVIEW
25.22.2 REVENUE ANALYSIS
25.22.3 GEOGRAPHIC PRESENCE
25.22.4 PRODUCT PORTFOLIO
25.22.5 RECENT DEVELOPMENTS
25.23 SUN PHARMACEUTICAL INDUSTRIES LTD.
25.23.1 COMPANY OVERVIEW
25.23.2 REVENUE ANALYSIS
25.23.3 GEOGRAPHIC PRESENCE
25.23.4 PRODUCT PORTFOLIO
25.23.5 RECENT DEVELOPMENTS
25.24 ZYDUS CADILA
25.24.1 COMPANY OVERVIEW
25.24.2 REVENUE ANALYSIS
25.24.3 GEOGRAPHIC PRESENCE
25.24.4 PRODUCT PORTFOLIO
25.24.5 RECENT DEVELOPMENTS
25.25 SUPERNUS PHARMACEUTICALS, INC.
25.25.1 COMPANY OVERVIEW
25.25.2 REVENUE ANALYSIS
25.25.3 GEOGRAPHIC PRESENCE
25.25.4 PRODUCT PORTFOLIO
25.25.5 RECENT DEVELOPMENTS
25.26 IMPEL NEUROPHARMA
25.26.1 COMPANY OVERVIEW
25.26.2 REVENUE ANALYSIS
25.26.3 GEOGRAPHIC PRESENCE
25.26.4 PRODUCT PORTFOLIO
25.26.5 RECENT DEVELOPMENTS
25.27 JOHNSON & JOHNSON SERVICES
25.27.1 COMPANY OVERVIEW
25.27.2 REVENUE ANALYSIS
25.27.3 GEOGRAPHIC PRESENCE
25.27.4 PRODUCT PORTFOLIO
25.27.5 RECENT DEVELOPMENTS
25.28 OTSUKA HOLDING CO., LTD
25.28.1 COMPANY OVERVIEW
25.28.2 REVENUE ANALYSIS
25.28.3 GEOGRAPHIC PRESENCE
25.28.4 PRODUCT PORTFOLIO
25.28.5 RECENT DEVELOPMENTS
25.29 AOBIOME
25.29.1 COMPANY OVERVIEW
25.29.2 REVENUE ANALYSIS
25.29.3 GEOGRAPHIC PRESENCE
25.29.4 PRODUCT PORTFOLIO
25.29.5 RECENT DEVELOPMENTS
25.3 ENDO PHARMACEUTICALS HOLDINGS INC
25.30.1 COMPANY OVERVIEW
25.30.2 REVENUE ANALYSIS
25.30.3 GEOGRAPHIC PRESENCE
25.30.4 PRODUCT PORTFOLIO
25.30.5 RECENT DEVELOPMENTS
26 RELATED REPORTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.