Europe Natural Killer (NK) Cell Therapeutics Market Analysis and Insights
The Europe natural killer (NK) cell therapeutics market comprises features such as the increasing need for better therapeutics options that will impact the launching of a new product by manufacturers into the market enhancing its demand as well as increasing investment in R&D leading to the market growth. Currently, various research studies are taking place which is expected to create a competitive advantage for manufacturers to develop new and innovative NK cell therapies, which is expected to provide various other opportunities for market growth. However, the lack of specificity and poor in-vivo survival of the cells, and the high cost associated with therapies are expected to restrain market growth in the forecast period.
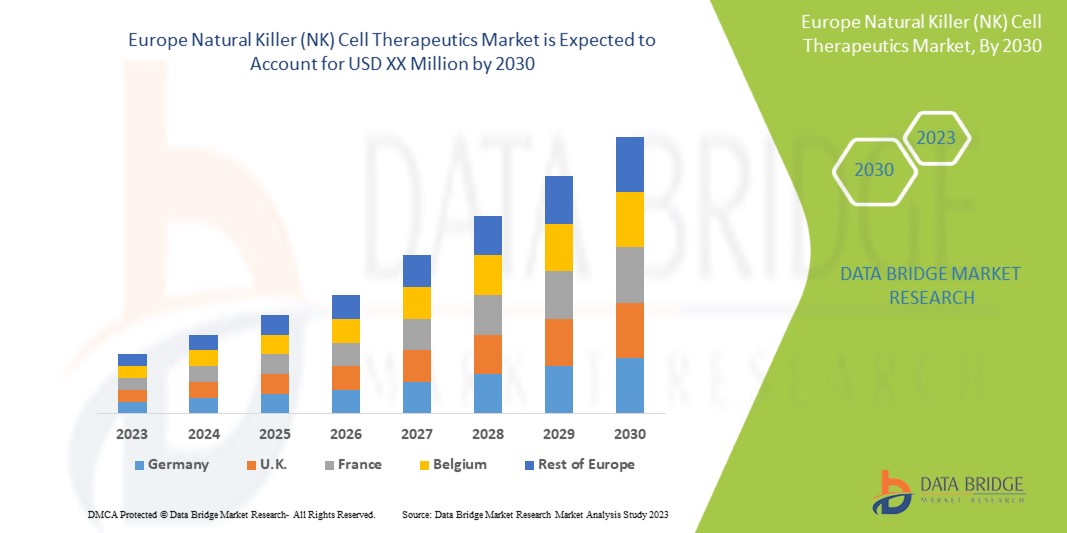
The Europe natural killer (NK) cell therapeutics market is supportive and aims to reduce the progression of the disease. Data Bridge Market Research analyzes that the Europe natural killer (NK) cell therapeutics market will grow at a CAGR of 43.3% during the forecast period of 2023 to 2030.
The Europe natural killer (NK) cell therapeutics market report provides details of market share, new developments, product pipeline analysis, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario, contact us for an analyst brief, our team will help you create a revenue-impact solution to achieve your desired goal.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in Million and Pricing in USD |
|
Segments Covered |
Therapeutics (NK Cell Therapies and NK Cell Directed Antibodies), Approaches (Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) and Bispecific Antibodies), Application (Cancer, Immunoproliferative Disorders, Acute Infectious Diseases, Gastrointestinal Diseases, and Others), End User (Research & Academic Institutes, Hospitals, and Specialty Clinics), Distribution Channel (Hospital Pharmacies, Direct Tender, and Others) |
|
Countries Covered |
Germany, France, Italy, U.K., Spain, Netherlands, Russia, Switzerland, Turkey, Austria, Norway, Hungary, Lithuania, Ireland, Poland, Luxembourg, rest of Europe |
|
Market Players Covered |
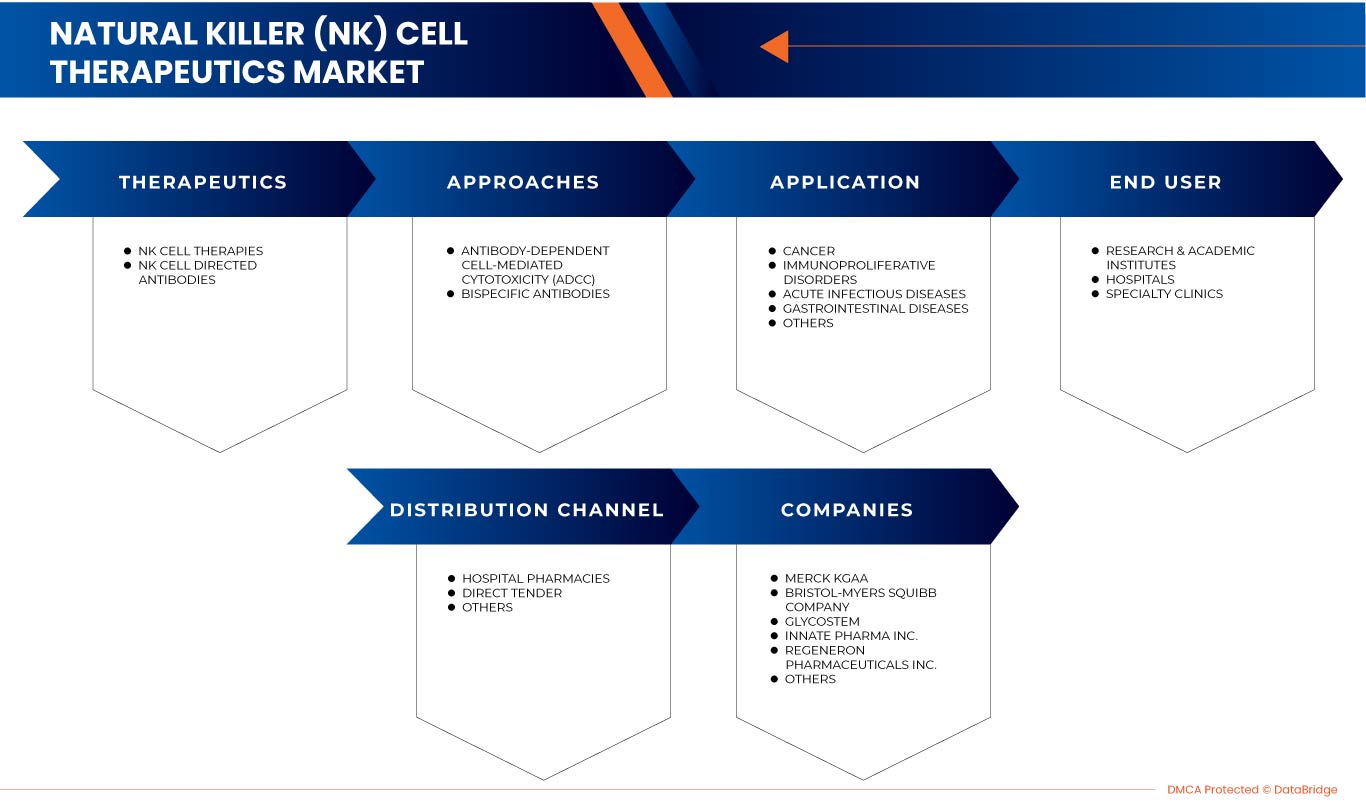
Merck KGaA, Bristol-Myers Squibb Company, Glycostem, Sanofi, Cytovia Therapeutics, ImmunityBio, Inc., Biohaven Pharmaceuticals, Fate Therapeutics, EMERcell, Phio Pharmaceuticals, PersonGen BioTherapeutics, Innate Pharma, Inc., INmuneBIO, Gamida Cell, Acepodia Inc., Affimed GmbH, Multimmune GmbH, iCell Gene Therapeutics, Takeda Pharmaceutical Company Limited, and Regeneron Pharmaceuticals Inc. among others |
Market Definition
Natural killer (NK) cells are large granular lymphocytes that have the ability to quickly respond to a pathological challenge. They are important components of the innate immune system and play an important role in generating an immune response against malignancies and infectious diseases caused by viral pathogens. They have been recognized to induce the direct killing of targeted cells involving cancer cells. They have the ability to recognize the multitude of infected cells without being dependent on a single antigen-expressing cell.
NK cells secrete cytokines and chemokines that recruit other immune cells such as T cells and B cells, which enhances the immune response against the tumor cells. As NK cells do not express T cell receptors, they induce a low risk of graft-versus-host disease. Moreover, iPSC cell lines derived NK cell therapy that is CAR-NK therapy overcomes the need for a long and complex manufacturing process that allows patients with aggressive tumors to afford tumor treatment as soon as possible. Along with this iPSC derived NK cells can be infused repeatedly, which enhances the immune response with dose regulation.
Europe Natural Killer (NK) Cell Therapeutics Market Dynamics
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
DRIVERS
- Increase in Patient Population with Chronic Diseases
Chronic diseases are diseases that generally occur in older adults that can be controlled but not cured such as heart disease, arthritis, diabetes, stroke, or cancer. They are a worldwide healthcare problem concern. The change in lifestyle along with physical inactivity, unhealthy diet, and tobacco use is increasing the burden of chronic diseases all over the world.
An increasing number of patients population with chronic diseases led the scientist and researchers to work on a new innovative therapeutic approach so as to provide patients with the best curative treatment option, which can be achieved by using NK cell therapy that has the potential to generate a strong immune response and has the anti-tumor capability as well.
- Rise in Awareness about Immunotherapies
As cancer is a devastating disease and available treatment options may cause the reoccurrence of tumors, the need for the best therapeutic approach is increasing day by day. Immunotherapies have attracted a lot of attention in recent years for the treatment of various types of malignancies. Among the immunotherapies, CAR–T cell therapies have been studied and were used as well but have several limitations, which led to the development of NK cell therapies. As a drug based on an NK cell therapeutic approach has not been approved yet, it thus needs to raise awareness among the population.
This is why several organizations and market players are launching a campaign to enhance awareness regarding NK cell therapies.
OPPORTUNITY
- Strategic Initiatives by Market Players
Various strategic initiatives are adopted by market players which involve expansion, acquisition, and collaboration among others. These initiatives help them to increase the company’s product portfolio leading to market expansion and hence enhancing the product demand among customers, which ultimately leads the players to earn maximum revenue.
As the demand for effective and affordable NK cell immunotherapy is increasing worldwide, these strategic initiatives are taken by top market players aimed at enhancing business operations and earning more profitability in the market.
RESTRAINT/CHALLENGE
- Lack of Specificity and Poor In Vivo Survival of the cells
Lack of specificity and poor in-vivo survival of NK cells poses a challenge in front of manufacturers dealing in the market. The lack of specificity of NK cells leads to targeting the wrong cells, which can lead to ineffective treatment. Along with this, the lack of specificity and poor in-vivo survival of NK cells also reduces the therapeutic effect of administered NK cell therapy.
Such problems decrease the credibility of therapy used in clinical trials and pose a challenge in front of manufacturers while getting approval.
Recent Developments
- In January 2022, Takeda acquired Adaptate biotherapeutics to develop novel gamma delta (γδ) T cell engager therapies targeting solid tumors. Through the acquisition, Takeda will obtain Adaptate’s antibody-based γδ T cell engager platform, including pre-clinical candidate and discovery pipeline programs. Adaptate’s γδ T cell engagers are designed to specifically modulate γδ T cell-mediated immune responses at tumor sites while sparing damage to healthy cells. This acquisition helped the company to focus on R&D.
- In December 2021, Nektar Therapeutics announced phase 1b data for novel T regulatory cell stimulator NKTR-358 (LY3471851) in patients with atopic dermatitis. NKTR-358 is designed to treat autoimmune and inflammatory conditions by correcting the immune system imbalance that results from increased levels of inflammatory T cells and reduced numbers and impaired function of immune regulating Treg cells. This has helped the company to focus on R&D for further phases.
Europe Natural Killer (NK) Cell Therapeutics Market Scope
The Europe natural killer (NK) cell therapeutics market is segmented into five notable segments based on therapeutics, approaches, application, end-user, and distribution channel. The growth among these segments will help you analyze niche pockets of growth and strategies to approach the market and determine the core application areas and the difference in the target markets.
Therapeutics
- NK Cell Therapies
- NK Cell Directed Antibodies
Based on therapeutics, the market is segmented into NK cell therapies and NK cell directed antibodies.
Approaches
- Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
- Bispecific Antibodies
Based on approaches, the market is segmented into antibody-dependent cell-mediated cytotoxicity (ADCC) and bispecific antibodies.
Application
- Cancer
- Immunoproliferative Disorders
- Acute Infectious Diseases
- Gastrointestinal Diseases
- Others
Based on application, the market is segmented into cancer, immunoproliferative disorders, acute infectious diseases, gastrointestinal diseases, and others.
End User
- Research & Academic Institutes
- Hospitals
- Specialty Clinics
Based on end user, the market is segmented into hospitals, specialty clinics, and research & academic institutes.
Distribution Channel
- Hospital Pharmacies
- Direct Tender
- Others
Based on distribution channel, the market is segmented into hospital pharmacies, direct tender, and others.
Europe Natural Killer (NK) Cell Therapeutics Market Regional Analysis/Insights
The Europe natural killer (NK) cell therapeutics market is categorized into five notable segments: therapeutics, approaches, application, end-user, and distribution channel.
The countries covered in this market report are Germany, France, Italy, U.K., Spain, Netherlands, Russia, Switzerland, Turkey, Austria, Norway, Hungary, Lithuania, Ireland, Poland, Luxembourg, rest of Europe.
The Germany dominates the Europe region due to the mass production of technologically advanced therapeutics and increasing demand from emerging markets and expansion of industries.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Europe brands and their challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data
Competitive Landscape and Europe Natural Killer (NK) Cell Therapeutics Market Share Analysis
The Europe natural killer (NK) cell therapeutics market competitive landscape provides details of competitors. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, Europe presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus related to the market.
Some of the major players operating in the Europe natural killer (NK) cell therapeutics market are, Merck KGaA,, Bristol-Myers Squibb Company, Glycostem, Sanofi, EMERcell, Phio Pharmaceuticals, Affimed GmbH, Multimmune GmbH, Takeda Pharmaceutical Company Limited, Regeneron Pharmaceuticals Inc., and Bellicum Pharmaceuticals, Inc.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.1.1 PATENT LANDSCAPE
6.1.2 USPTO NUMBER
6.1.3 PATENT EXPIRY
6.1.4 EPIO NUMBER
6.1.5 PATENT STRENGTH AND QUALITY
6.1.6 PATENT CLAIMS
6.1.7 PATENT CITATIONS
6.1.8 PATENT LITIGATION AND LICENSING
6.1.9 FILE OF PATENT
6.1.10 PATENT RECEIVED CONTRIES
6.1.11 TECHNOLOGY BACKGROUND
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH SPECIALIST
6.8 OTHER KOL SNAPSHOTS
7 EPIDEMIOLOGY
7.1 INCIDENCE OF ALL BY GENDER
7.2 TREATMENT RATE
7.3 MORTALITY RATE
7.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
7.5 PATIENT TREATMENT SUCCESS RATES
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 REGULATORY FRAMEWORK
9.1 REGULATORY APPROVAL PROCESS
9.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
9.3 REGULATORY APPROVAL PATHWAYS
9.4 LICENSING AND REGISTRATION
9.5 POST-MARKETING SURVEILLANCE
9.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
10 PIPELINE ANALYSIS
10.1 CLINICAL TRIALS AND PHASE ANALYSIS
10.2 DRUG THERAPY PIPELINE
10.3 PHASE III CANDIDATES
10.4 PHASE II CANDIDATES
10.5 PHASE I CANDIDATES
10.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 EUROPE CLINICAL TRIAL MARKET FOR NATURAL KILLER (NK) CELL THERAPEUTICS MARKET
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
11 MARKETED DRUG ANALYSIS
11.1 DRUG
11.1.1 BRAND NAME
11.1.2 GENERICS NAME
11.2 THERAPEUTIC INDICTION
11.3 PHARMACOLOGICAL CLASS OF THE DRUG
11.4 DRUG PRIMARY INDICATION
11.5 MARKET STATUS
11.6 MEDICATION TYPE
11.7 DRUG DOSAGES FORM
11.8 DOSAGES AVAILABILITY
11.9 DRUG ROUTE OF ADMINISTRATION
11.1 DOSING FREQUENCY
11.11 DRUG INSIGHT
11.12 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
11.12.1 FORECAST MARKET OUTLOOK
11.12.2 CROSS COMPETITION
11.12.3 THERAPEUTIC PORTFOLIO
11.12.4 CURRENT DEVELOPMENT SCENARIO
12 MARKET ACCESS
12.1 10-YEAR MARKET FORECAST
12.2 CLINICAL TRIAL RECENT UPDATES
12.3 ANNUAL NEW FDA APPROVED DRUGS
12.4 DRUGS MANUFACTURER AND DEALS
12.5 MAJOR DRUG UPTAKE
12.6 CURRENT TREATMENT PRACTICES
12.7 IMPACT OF UPCOMING THERAPY
13 R & D ANALYSIS
13.1 COMPARATIVE ANALYSIS
13.2 DRUG DEVELOPMENTAL LANDSCAPE
13.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
13.4 THERAPEUTIC ASSESSMENT
13.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
14 MARKET OVERVIEW
14.1 DRIVERS
14.2 RESTRAINTS
14.3 OPPORTUNITIES
14.4 CHALLENGES
15 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, BY SOURCE
15.1 OVERVIEW
15.2 AUTOLOGOUS CELLS
15.2.1 NATURAL KILLER (NK) CELL
15.2.2 CAR (CHIMERIC ANTIGEN RECEPTOR) NK CELLS
15.3 ALLOGENIC CELLS
15.3.1 NATURAL KILLER (NK) CELL
15.3.2 CAR (CHIMERIC ANTIGEN RECEPTOR) NK CELLS
16 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, BY TYPE
16.1 OVERVIEW
16.2 NATURAL KILLER (NK) CELL
16.2.1 BY ORIGIN
16.2.1.1. DONOR-DERIVED NK
16.2.1.1.1. UMBILICAL CORD
16.2.1.1.2. PLACENTA
16.2.1.2. IPSC-NK CELLS
16.2.1.3. CYTOKINE-INDUCED
16.2.1.4. OTHERS
16.2.1.4.1. PB-NK CELLS
16.2.1.4.2. UB-NK CELLS
16.2.1.4.3. NK92 CELLS
16.2.2 BY THERAPEUTICS
16.2.2.1. NK CELL THERAPIES
16.2.2.2. NK CELL DIRECTED ANTIBODIES
16.2.2.3. OTHERS
16.2.3 BY APPROACHES
16.2.3.1. BISPECIFIC ANTIBODIES
16.2.3.2. ANTIBODY-DEPENDENT CELL-MEDIATED CYTOTOXICITY (ADCC)
16.2.3.3. CELL-AND-GENE-THERAPY WITH NK CELLS
16.2.3.4. OTHERS
16.3 CAR (CHIMERIC ANTIGEN RECEPTOR) NK CELLS
16.3.1 CAR-NK CELLS
16.3.1.1. BY SOURCE
16.3.1.1.1. AUTOLOGOUS CAR-NK CELLS
16.3.1.1.2. ALLOGENEIC CAR-NK CELLS
16.3.1.2. BY NK CELLS ENGINEERED
16.3.1.2.1. PERIPHERAL BLOOD
16.3.1.2.2. UMBILICAL CORD BLOOD
16.3.1.2.3. CELL LINES (NK-92)
16.3.1.2.4. IPSC-DERIVED CAR NK
16.3.1.3. BY STRUCTURE
16.3.1.3.1. FIRST-GENERATION
16.3.1.3.2. SECOND-GENERATION
16.3.1.3.3. THIRD-GENERATION
16.3.1.4. BY BRAND (PIPELINE)
16.3.1.4.1. AB-205
16.3.1.4.2. AB-201
16.3.1.4.3. TAK-007
16.3.1.4.4. CYTO NK-102
16.3.1.4.5. OTHERS
17 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, BY THERAPEUTICS
17.1 OVERVIEW
17.2 FIRST-GENERATION
17.3 SECOND-GENERATION
17.4 THIRD-GENERATION
17.5 FOURTH-GENERATION
18 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, BY AGE GROUP
18.1 OVERVIEW
18.2 PEDIATRIC
18.3 ADULTS
18.4 GERIATRIC
19 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, BY GENDER
19.1 OVERVIEW
19.2 MALE
19.2.1 CHILDREN (0-15)
19.2.2 ADULT (16- 64)
19.2.3 SENIORS (65 AND ABOVE)
19.3 FEMALE
19.3.1 CHILDREN (0-15)
19.3.2 ADULT (16- 64)
19.3.3 SENIORS (65 AND ABOVE)
20 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, BY APPLICATION
20.1 OVERVIEW
20.2 FOLLICULAR LYMPHOMA
20.3 DIFFUSE LARGE B-CELL LYMPHOMA
20.4 ACUTE LYMPHOBLASTIC LEUKEMIA (ALL)
20.5 MANTLE CELL LYMPHOMA
20.6 MULTIPLE MYELOMA
20.7 HEMATOLOGIC MALIGNANCIES
20.7.1 LEUKEMIA
20.7.2 LYMPHOMA
20.7.3 MYELOMA
20.7.4 OTHERS
20.8 LUNG CANCER
20.9 BREAST CANCER
20.1 GASTRIC CANCER
20.11 PANCREATIC CANCER
20.12 CHRONIC LYMPHOCYTIC LEUKEMIA
20.13 OTHERS
21 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITALS
21.2.1 PUBLIC
21.2.2 PRIVATE
21.3 SPECIALTY CLINICS
21.4 ONCOLOGY CENTRES
21.5 RESEARCH & ACADEMIC INSTITUTES
21.6 OTHERS
22 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, BY DISTRIBUTION CHANNEL
22.1 OVERVIEW
22.2 DIRECT TENDER
22.3 RETAIL SALES
22.4 OTHERS
23 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: EUROPE
23.2 MERGERS & ACQUISITIONS
23.3 NEW PRODUCT DEVELOPMENT & APPROVALS
23.4 EXPANSIONS
23.5 REGULATORY CHANGES
23.6 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, BY REGION
EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, (EUROPE SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
24.1 EUROPE
24.1.1 GERMANY
24.1.2 FRANCE
24.1.3 U.K.
24.1.4 HUNGARY
24.1.5 LITHUANIA
24.1.6 AUSTRIA
24.1.7 IRELAND
24.1.8 NORWAY
24.1.9 POLAND
24.1.10 ITALY
24.1.11 SPAIN
24.1.12 RUSSIA
24.1.13 TURKEY
24.1.14 NETHERLANDS
24.1.15 SWITZERLAND
24.1.16 REST OF EUROPE
24.2 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
25 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, SWOT AND DBMR ANALYSIS
26 EUROPE NATURAL KILLER (NK) CELL THERAPEUTICS MARKET, COMPANY PROFILE
(SWOT AND DBMR ANALYSIS OF TOP 5 COMPANIES WILL BE PROVIDED)
26.1 SHORELINE BIOSCIENCES
26.1.1 COMPANY OVERVIEW
26.1.2 REVENUE ANALYSIS
26.1.3 GEOGRAPHIC PRESENCE
26.1.4 RECENT DEVELOPMENTS
26.2 HEBECELL CORP + ANKARYS THERAPEUTICS + APPLIED STEMCELL
26.2.1 COMPANY OVERVIEW
26.2.2 REVENUE ANALYSIS
26.2.3 GEOGRAPHIC PRESENCE
26.2.4 PRODUCT PORTFOLIO
26.2.5 RECENT DEVELOPMENTS
26.3 CYTOVIA THERAPEUTICS
26.3.1 COMPANY OVERVIEW
26.3.2 REVENUE ANALYSIS
26.3.3 GEOGRAPHIC PRESENCE
26.3.4 RECENT DEVELOPMENTS
26.4 NKARTA, INC.
26.4.1 COMPANY OVERVIEW
26.4.2 REVENUE ANALYSIS
26.4.3 GEOGRAPHIC PRESENCE
26.4.4 PRODUCT PORTFOLIO
26.4.5 RECENT DEVELOPMENTS
26.5 IMMUNITYBIO, INC. + NANTKWEST
26.5.1 COMPANY OVERVIEW
26.5.2 REVENUE ANALYSIS
26.5.3 GEOGRAPHIC PRESENCE
26.5.4 PRODUCT PORTFOLIO
26.5.5 RECENT DEVELOPMENTS
26.6 TAKEDA PHARMACEUTICAL COMPANY LIMITED
26.6.1 COMPANY OVERVIEW
26.6.2 REVENUE ANALYSIS
26.6.3 GEOGRAPHIC PRESENCE
26.6.4 PRODUCT PORTFOLIO
26.6.5 RECENT DEVELOPMENTS
26.7 ABIVAX
26.7.1 COMPANY OVERVIEW
26.7.2 REVENUE ANALYSIS
26.7.3 GEOGRAPHIC PRESENCE
26.7.4 PRODUCT PORTFOLIO
26.7.5 RECENT DEVELOPMENTS
26.8 AFFIMED N.V. + ARTIVA BIOTHERAPEUTICS INC.
26.8.1 COMPANY OVERVIEW
26.8.2 REVENUE ANALYSIS
26.8.3 GEOGRAPHIC PRESENCE
26.8.4 PRODUCT PORTFOLIO
26.8.5 RECENT DEVELOPMENTS
26.9 FORTRESS BIOTECH, INC.
26.9.1 COMPANY OVERVIEW
26.9.2 REVENUE ANALYSIS
26.9.3 GEOGRAPHIC PRESENCE
26.9.4 PRODUCT PORTFOLIO
26.9.5 RECENT DEVELOPMENTS
26.1 CANTARGIA AB
26.10.1 COMPANY OVERVIEW
26.10.2 REVENUE ANALYSIS
26.10.3 GEOGRAPHIC PRESENCE
26.10.4 PRODUCT PORTFOLIO
26.10.5 RECENT DEVELOPMENTS
26.11 FATE THERAPEUTICS
26.11.1 COMPANY OVERVIEW
26.11.2 REVENUE ANALYSIS
26.11.3 GEOGRAPHIC PRESENCE
26.11.4 PRODUCT PORTFOLIO
26.11.5 RECENT DEVELOPMENTS
26.12 GLYCOSTEM
26.12.1 COMPANY OVERVIEW
26.12.2 REVENUE ANALYSIS
26.12.3 GEOGRAPHIC PRESENCE
26.12.4 PRODUCT PORTFOLIO
26.12.5 RECENT DEVELOPMENTS
26.13 SORRENTO THERAPEUTICS, INC.
26.13.1 COMPANY OVERVIEW
26.13.2 REVENUE ANALYSIS
26.13.3 GEOGRAPHIC PRESENCE
26.13.4 PRODUCT PORTFOLIO
26.13.5 RECENT DEVELOPMENTS
26.14 ALL LIFE ADVANCED IMMUNOLOGY
26.14.1 COMPANY OVERVIEW
26.14.2 REVENUE ANALYSIS
26.14.3 GEOGRAPHIC PRESENCE
26.14.4 PRODUCT PORTFOLIO
26.14.5 RECENT DEVELOPMENTS
26.15 EXACIS BIOTHERAPEUTICS, INC.
26.15.1 COMPANY OVERVIEW
26.15.2 REVENUE ANALYSIS
26.15.3 GEOGRAPHIC PRESENCE
26.15.4 PRODUCT PORTFOLIO
26.15.5 RECENT DEVELOPMENTS
26.16 NKMAX CO., LTD.
26.16.1 COMPANY OVERVIEW
26.16.2 REVENUE ANALYSIS
26.16.3 GEOGRAPHIC PRESENCE
26.16.4 PRODUCT PORTFOLIO
26.16.5 RECENT DEVELOPMENTS
26.17 NEKTAR
26.17.1 COMPANY OVERVIEW
26.17.2 REVENUE ANALYSIS
26.17.3 GEOGRAPHIC PRESENCE
26.17.4 PRODUCT PORTFOLIO
26.17.5 RECENT DEVELOPMENTS
26.18 GAMIDA CELL LTD
26.18.1 COMPANY OVERVIEW
26.18.2 REVENUE ANALYSIS
26.18.3 GEOGRAPHIC PRESENCE
26.18.4 PRODUCT PORTFOLIO
26.18.5 RECENT DEVELOPMENTS
26.19 MULTIMMUNE GMBH
26.19.1 COMPANY OVERVIEW
26.19.2 REVENUE ANALYSIS
26.19.3 GEOGRAPHIC PRESENCE
26.19.4 PRODUCT PORTFOLIO
26.19.5 RECENT DEVELOPMENTS
26.2 XNK THERAPEUTICS
26.20.1 COMPANY OVERVIEW
26.20.2 REVENUE ANALYSIS
26.20.3 GEOGRAPHIC PRESENCE
26.20.4 PRODUCT PORTFOLIO
26.20.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
27 RELATED REPORTS
28 CONCLUSION
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.