Global Adalimumab Biosimilar Market
Market Size in USD Million
CAGR :
% 
 USD
598.30 Million
USD
3,431.48 Million
2022
2030
USD
598.30 Million
USD
3,431.48 Million
2022
2030
| 2023 –2030 | |
| USD 598.30 Million | |
| USD 3,431.48 Million | |
|
|
|
|
Adalimumab Biosimilar Market Analysis and Size
According to GLOBOCAN 2020, there will be 2,281,658 new cancer cases and nearly 612,390 deaths in the United States in 2020. Furthermore, key market participants predicted market growth over the forecast period through various strategic activities such as product launches, mergers, and acquisitions. For instance, in July 2021, the FDA approved Viatris Inc.'s (formerly Mylan Pharmaceuticals Inc.) SEMGLEE (insulin-glargine-yfgn), a biosimilar to LANTUS (insulin glargine).
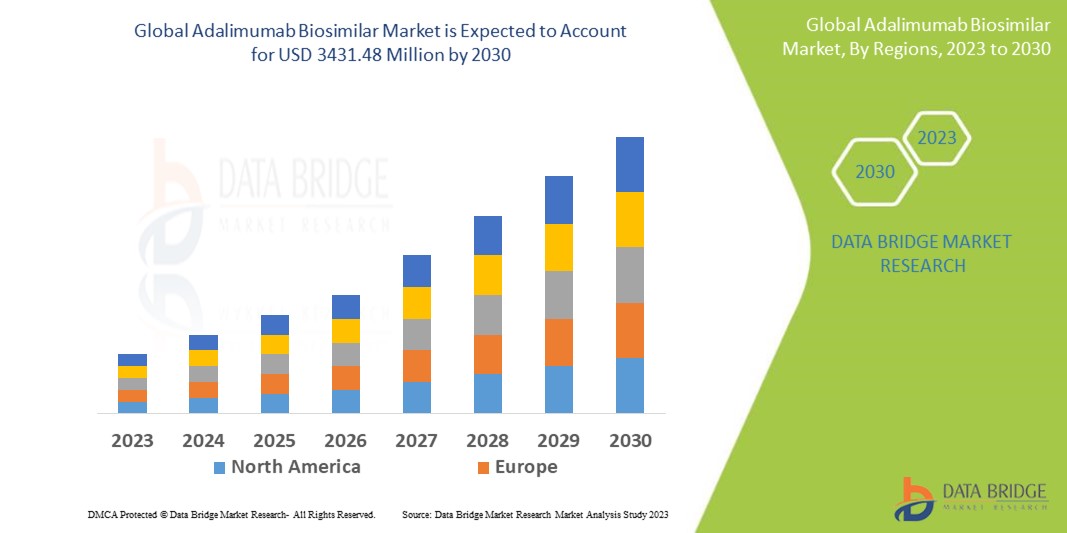
Data Bridge Market Research analyses that the adalimumab biosimilar market which is USD 598.30 million in 2022, is expected to reach USD 3431.48 million by 2030, at a CAGR of 24.4% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Adalimumab Biosimilar Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product (Exemptia, Adalirel, Cipleumab, Others), Distribution Channel (Hospitals Pharmacies, Retail Pharmacies, Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Alfred E. Tiefenbacher (GmbH & Co. KG) (Germany), Amgen Inc. (U.S.), Boehringer Ingelheim International GmbH (Germany), Glenmark (India), Zydus Group (India), Torrent Pharmaceuticals Ltd. (India), Reliance Life Sciences (India), Emcure Pharmaceuticals Ltd (India), Cipla Inc. (India), Hetero (India), AET BioTech (Germany), Coherus Biosciences (U.S.), Fujifilm Kyowa Kirin Biologics Co. Ltd. (Japan), Momenta Pharmaceuticals (U.S.), Oncobiologics (U.S.), Pfizer Inc. (U.S.), Samsung Bioepsis (South Korea), Sandoz International GmbH (Switzerland) |
|
Market Opportunities |
|
Market Definition
Adalimumab, a monoclonal antibody, is used to treat autoimmune diseases such as rheumatoid arthritis and ulcerative colitis, among others. This medication helps to prevent future joint damage and maintain joint function by reducing joint swelling. As the global prevalence of arthritis rises, the market for adalimumab biosimilars is expected to grow during the forecast period.
Adalimumab Biosimilar Market Dynamics
Drivers
- Rise in biologic drugs
Several blockbuster biologic drugs from major pharmaceutical companies, including Truvada, Chantix, Forteo, Ciprodex, Afinitor, and many others, will lose US exclusivity in 2020. Several existing biological drugs, such as Erbitux, Avastin, and Orencia, will have their patents expire in the coming decade, providing an opportunity for many innovator companies as well as generic manufacturers to offer services specifically tailored toward biosimilars. Furthermore, the cost-effective nature of biosimilars, rising acceptance and adoption by various stakeholders, the need for diversification in technology and business models, and the growing prevalence of chronic diseases are expected to drive the global biosimilar market.
Opportunities
- Rising cancer cases
Cancer's growing burden and rising death toll necessitate the need for affordable treatment, boosting the growth of the biosimilar market. According to GLOBOCAN 2020, there will be 2,281,658 new cancer cases and nearly 612,390 deaths in the United States in 2020. Furthermore, key market participants predicted market growth during the forecast period through a variety of strategic activities such as product launches, mergers, and acquisitions. For instance, in July 2021, the FDA approved Viatris Inc.'s (formerly Mylan Pharmaceuticals Inc.) SEMGLEE (insulin-glargine-yfgn), a biosimilar to LANTUS (insulin glargine).
Restraints/Challenges
- High cost
The high cost associated with biosimilars will obstruct the market's growth rate. However, the global adalimumab biosimilar market is hampered by a scarcity of skilled specialists. Furthermore, factors such as high temperature sensitivity, lack of efficacy and safety, and high manufacturing costs associated with generic drugs are expected to limit market growth between 2022 and 2030.
This adalimumab biosimilar market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the adalimumab biosimilar market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global Adalimumab Biosimilar Market Scope
The adalimumab biosimilar market is segmented on the basis of product and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Exemptia
- Adalirel
- Cipleumab
- Others
Distribution Channel
- Hospitals Pharmacies
- Retail Pharmacies
- Others
Adalimumab Biosimilar Market Regional Analysis/Insights
The adalimumab biosimilar market is analyzed and market size insights and trends are provided by country, product and distribution channel as referenced above.
The countries covered in the adalimumab biosimilar market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the adalimumab biosimilar market due to the proximity of multiple extensive experimentation laboratories.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030 because of the province's extensive commercial advancements and growing biotechnology corporations.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Adalimumab Biosimilar Market Share Analysis
The adalimumab biosimilar market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to adalimumab biosimilar market.
Some of the major players operating in the adalimumab biosimilar market are:
- Alfred E. Tiefenbacher (GmbH & Co. KG) (Germany)
- Amgen Inc. (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- Glenmark (India)
- Zydus Group (India)
- Torrent Pharmaceuticals Ltd. (India)
- Reliance Life Sciences (India)
- Emcure Pharmaceuticals Ltd (India)
- Cipla Inc. (India)
- Hetero (India)
- AET BioTech (Germany)
- Coherus Biosciences (U.S.)
- Fujifilm Kyowa Kirin Biologics Co. Ltd. (Japan)
- Momenta Pharmaceuticals (U.S.)
- Oncobiologics (U.S.)
- Pfizer Inc. (U.S.)
- Samsung Bioepsis (South Korea)
- Sandoz International GmbH (Switzerland)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ADALIMUMAB BIOSIMILAR MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ADALIMUMAB BIOSIMILAR MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ADALIMUMAB BIOSIMILAR MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 EPIDEMIOLOGY
11.1 INCIDENCE OF ALL BY GENDER
11.2 TREATMENT RATE
11.3 MORTALITY RATE
11.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
11.5 PATIENT TREATMENT SUCCESS RATES
12 REGULATORY COMPLIANCE
12.1 REGULATORY AUTHORITIES
12.2 REGULATORY CLASSIFICATIONS
12.2.1 CLASS I
12.2.2 CLASS II
12.2.3 CLASS III
12.3 REGULATORY SUBMISSIONS
12.4 INTERNATIONAL HARMONIZATION
12.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
12.6 REGULATORY CHALLENGES AND STRATEGIES
13 PIPELINE ANALYSIS
13.1 CLINICAL TRIALS AND PHASE ANALYSIS
13.2 DRUG THERAPY PIPELINE
13.3 PHASE III CANDIDATES
13.4 PHASE II CANDIDATES
13.5 PHASE I CANDIDATES
13.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR ADALIMUMAB BIOSIMILAR MARKET
Company Name Product Name
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE FOR ADALIMUMAB BIOSIMILAR MARKET
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved but Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE FOR ADALIMUMAB BIOSIMILAR MARKET
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE FOR ADALIMUMAB BIOSIMILAR MARKET
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR ADALIMUMAB BIOSIMILAR MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
14 REIMBURSEMENT FRAMEWORK
15 OPPUTUNITY MAP ANALYSIS
16 VALUE CHAIN ANALYSIS
17 HEALTHCARE ECONOMY
17.1 HEALTHCARE EXPENDITURE
17.2 CAPITAL EXPENDITURE
17.3 CAPEX TRENDS
17.4 CAPEX ALLOCATION
17.5 FUNDING SOURCES
17.6 INDUSTRY BENCHMARKS
17.7 GDP RATION IN OVERALL GDP
17.8 HEALTHCARE SYSTEM STRUCTURE
17.9 GOVERNMENT POLICIES
17.1 ECONOMIC DEVELOPMENT
18 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, BY PRODUCT
18.1 OVERVIEW
18.2 MARKETED/APPROVED
18.2.1 EXEMPTIA
18.2.1.1. MARKET VALUE (USD MN)
18.2.1.2. MARKET VOLUME (STANDARD UNIT)
18.2.1.3. AVERAGE SELLING PRICE (USD)
18.2.2 ADALIREL
18.2.2.1. MARKET VALUE (USD MN)
18.2.2.2. MARKET VOLUME (STANDARD UNIT)
18.2.2.3. AVERAGE SELLING PRICE (USD)
18.2.3 CIPLEUMAB
18.2.3.1. MARKET VALUE (USD MN)
18.2.3.2. MARKET VOLUME (STANDARD UNIT)
18.2.3.3. AVERAGE SELLING PRICE (USD)
18.2.4 AMJEVITA
18.2.4.1. MARKET VALUE (USD MN)
18.2.4.2. MARKET VOLUME (STANDARD UNIT)
18.2.4.3. AVERAGE SELLING PRICE (USD)
18.2.5 YUFLYMA (ADALIMUMAB-AATY)
18.2.5.1. MARKET VALUE (USD MN)
18.2.5.2. MARKET VOLUME (STANDARD UNIT)
18.2.5.3. AVERAGE SELLING PRICE (USD)
18.2.6 CYLTEZO (ADALIMUMAB-ADBM)
18.2.6.1. MARKET VALUE (USD MN)
18.2.6.2. MARKET VOLUME (STANDARD UNIT)
18.2.6.3. AVERAGE SELLING PRICE (USD)
18.2.7 HADLIMA (ADALIMUMAB-BWWD)
18.2.7.1. MARKET VALUE (USD MN)
18.2.7.2. MARKET VOLUME (STANDARD UNIT)
18.2.7.3. AVERAGE SELLING PRICE (USD)
18.2.8 SIMLANDI (ADALIMUMAB-RYVK)
18.2.8.1. MARKET VALUE (USD MN)
18.2.8.2. MARKET VOLUME (STANDARD UNIT)
18.2.8.3. AVERAGE SELLING PRICE (USD)
18.2.9 ABRILADA (ADALIMUMAB-AFZB)
18.2.9.1. MARKET VALUE (USD MN)
18.2.9.2. MARKET VOLUME (STANDARD UNIT)
18.2.9.3. AVERAGE SELLING PRICE (USD)
18.2.10 HULIO (ADALIMUMAB-FKJP)
18.2.10.1. MARKET VALUE (USD MN)
18.2.10.2. MARKET VOLUME (STANDARD UNIT)
18.2.10.3. AVERAGE SELLING PRICE (USD)
18.2.11 IDACIO (ADALIMUMAB-AACF)
18.2.11.1. MARKET VALUE (USD MN)
18.2.11.2. MARKET VOLUME (STANDARD UNIT)
18.2.11.3. AVERAGE SELLING PRICE (USD)
18.2.12 HYRIMOZ (ADALIMUMAB-ADAZ)
18.2.12.1. MARKET VALUE (USD MN)
18.2.12.2. MARKET VOLUME (STANDARD UNIT)
18.2.12.3. AVERAGE SELLING PRICE (USD)
18.2.13 YUSIMRY (ADALIMUMAB-AQVH)
18.2.13.1. MARKET VALUE (USD MN)
18.2.13.2. MARKET VOLUME (STANDARD UNIT)
18.2.13.3. AVERAGE SELLING PRICE (USD)
18.2.14 IMRALDI (ADALIMUMAB)
18.2.14.1. MARKET VALUE (USD MN)
18.2.14.2. MARKET VOLUME (STANDARD UNIT)
18.2.14.3. AVERAGE SELLING PRICE (USD)
18.2.15 QLETLI
18.2.15.1. MARKET VALUE (USD MN)
18.2.15.2. MARKET VOLUME (STANDARD UNIT)
18.2.15.3. AVERAGE SELLING PRICE (USD)
18.2.16 XELENKA
18.2.16.1. MARKET VALUE (USD MN)
18.2.16.2. MARKET VOLUME (STANDARD UNIT)
18.2.16.3. AVERAGE SELLING PRICE (USD)
18.2.17 SULINNO
18.2.17.1. MARKET VALUE (USD MN)
18.2.17.2. MARKET VOLUME (STANDARD UNIT)
18.2.17.3. AVERAGE SELLING PRICE (USD)
18.2.18 OTHERS
18.3 PIPELINE
18.3.1 BCD-057
18.3.2 M923
18.3.3 CINNORA
18.3.4 HS016
18.3.5 HLX03
18.3.6 PBP1502
18.3.7 ONS-3010
18.3.8 OTHERS
19 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, BY FORMS
19.1 OVERVIEW
19.2 PREFILLED SYRINGES
19.2.1 10 MG
19.2.2 20 MG
19.2.3 40 MG
19.2.4 80 MG
19.3 PREFILLED PEN
19.4 AUTOINJECTOR PENS
19.4.1 CLICK AUTOINJECTOR
19.4.2 PUSH AUTOINJECTOR
19.5 VIALS
19.6 OTHERS
20 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, BY INDICATION
20.1 OVERVIEW
20.2 RHEUMATOID ARTHRITIS
20.2.1 MARKETED/APPROVED
20.2.2 PIPELINE
20.3 JUVENILE IDIOPATHIC ARTHRITIS
20.3.1 MARKETED/APPROVED
20.3.2 PIPELINE
20.4 PSORIATIC ARTHRITIS
20.4.1 MARKETED/APPROVED
20.4.2 PIPELINE
20.5 ANKYLOSING SPONDYLITIS
20.5.1 MARKETED/APPROVED
20.5.2 PIPELINE
20.6 CROHN’S DISEASE
20.6.1 MARKETED/APPROVED
20.6.2 PIPELINE
20.7 CHRONIC PLAQUE PSORIASIS
20.7.1 MARKETED/APPROVED
20.7.2 PIPELINE
20.8 ULCERATIVE COLITIS
20.8.1 MARKETED/APPROVED
20.8.2 PIPELINE
20.9 HIDRADENITIS SUPPURATIVA
20.9.1 MARKETED/APPROVED
20.9.2 PIPELINE
20.1 NON-INFECTIOUS INTERMEDIATE
20.10.1 MARKETED/APPROVED
20.10.2 PIPELINE
20.11 OTHERS
21 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, BY DOSAGE STRENGTH
21.1 OVERVIEW
21.2 40MG/0.4MLG
21.3 80MG/0.8MLG
21.4 20MG/0.2MLG
21.5 10MG/0.1MLG
21.6 OTHERS
22 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, ROUTE OF ADMINISTRATION
22.1 OVERVIEW
22.2 INTRAVENEOUS
22.3 SUBCUTANEOUS
22.4 OTHERS
23 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, BY POPULATION TYPE
23.1 OVERVIEW
23.2 PEDIATRIC
23.3 ADULTS
23.4 GERIATRIC
24 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, BY GENDER
24.1 OVERVIEW
24.2 MALE
24.2.1 PEDIATRIC
24.2.2 ADULTS
24.2.3 GERIATRIC
24.3 FEMALE
24.3.1 PEDIATRIC
24.3.2 ADULTS
24.3.3 GERIATRIC
25 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, BY END USER
25.1 OVERVIEW
25.2 HOSPITALS
25.2.1 PUBLIC
25.2.2 PRIVATE
25.3 SPECIALTY CLINICS
25.4 HOME HEALTHCARE
25.5 ACADEMIC AND RESEARCH INSTITUTE
25.6 OTHERS
26 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, BY DISTRIBUTION CHANNEL
26.1 OVERVIEW
26.2 DIRECT TENDER
26.3 RETAIL SALES
26.3.1 HOSPITAL PHARMACIES
26.3.2 DRUG STORES
26.3.3 PHARMACY CHAINS
26.3.4 ONLINE PHARMACIES
26.3.4.1. E-PHARMACY COMPANIES
26.3.4.2. COMPANY OWNED E-PHARMACY
26.4 OTHERS
27 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, BY GEOGRAPHY
GLOBAL ADALIMUMAB BIOSIMILAR MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
27.1 NORTH AMERICA
27.1.1 U.S.
27.1.2 CANADA
27.1.3 MEXICO
27.1.4 DOMINICAN REPUBLIC
27.1.5 JAMAICA
27.1.6 PANAMA
27.2 EUROPE
27.2.1 GERMANY
27.2.2 FRANCE
27.2.3 U.K.
27.2.4 HUNGARY
27.2.5 LITHUANIA
27.2.6 AUSTRIA
27.2.7 IRELAND
27.2.8 NORWAY
27.2.9 POLAND
27.2.10 ITALY
27.2.11 SPAIN
27.2.12 RUSSIA
27.2.13 TURKEY
27.2.14 NETHERLANDS
27.2.15 SWITZERLAND
27.2.16 REST OF EUROPE
27.3 ASIA-PACIFIC
27.3.1 JAPAN
27.3.2 CHINA
27.3.3 TAIWAN
27.3.4 SOUTH KOREA
27.3.5 INDIA
27.3.6 AUSTRALIA
27.3.7 SINGAPORE
27.3.8 THAILAND
27.3.9 MALAYSIA
27.3.10 INDONESIA
27.3.11 PHILIPPINES
27.3.12 VIETNAM
27.3.13 REST OF ASIA-PACIFIC
27.4 SOUTH AMERICA
27.4.1 BRAZIL
27.4.2 ECUADOR
27.4.3 CHILE
27.4.4 COLOMBIA
27.4.5 VENEZUELA
27.4.6 ARGENTINA
27.4.7 PERU
27.4.8 CURAÇAO
27.4.9 PARAGUAY
27.4.10 URUGUAY
27.4.11 TRINIDAD AND TOBAGO
27.4.12 REST OF SOUTH AMERICA
27.5 MIDDLE EAST AND AFRICA
27.5.1 SOUTH AFRICA
27.5.2 SAUDI ARABIA
27.5.3 UAE
27.5.4 EGYPT
27.5.5 KUWAIT
27.5.6 ISRAEL
27.5.7 BOLIVIA
27.5.8 REST OF MIDDLE EAST AND AFRICA
27.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
28 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, SWOT AND DBMR ANALYSIS
29 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, COMPANY LANDSCAPE
29.1 COMPANY SHARE ANALYSIS: GLOBAL
29.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
29.3 COMPANY SHARE ANALYSIS: EUROPE
29.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
29.5 MERGERS & ACQUISITIONS
29.6 NEW PRODUCT DEVELOPMENT & APPROVALS
29.7 EXPANSIONS
29.8 REGULATORY CHANGES
29.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
30 GLOBAL ADALIMUMAB BIOSIMILAR MARKET, COMPANY PROFILE
30.1 MARKETED COMPANY
30.1.1 AMGEN INC.
30.1.1.1. COMPANY OVERVIEW
30.1.1.2. REVENUE ANALYSIS
30.1.1.3. GEOGRAPHIC PRESENCE
30.1.1.4. PRODUCT PORTFOLIO
30.1.1.5. RECENT DEVELOPMENTS
30.1.2 BOEHRINGER INGELHEIM GMBH
30.1.2.1. COMPANY OVERVIEW
30.1.2.2. REVENUE ANALYSIS
30.1.2.3. GEOGRAPHIC PRESENCE
30.1.2.4. PRODUCT PORTFOLIO
30.1.2.5. RECENT DEVELOPMENTS
30.1.3 SANDOZ INC. (NOVARTIS AG)
30.1.3.1. COMPANY OVERVIEW
30.1.3.2. REVENUE ANALYSIS
30.1.3.3. GEOGRAPHIC PRESENCE
30.1.3.4. PRODUCT PORTFOLIO
30.1.3.5. RECENT DEVELOPMENTS
30.1.4 BIOGEN
30.1.4.1. COMPANY OVERVIEW
30.1.4.2. REVENUE ANALYSIS
30.1.4.3. GEOGRAPHIC PRESENCE
30.1.4.4. PRODUCT PORTFOLIO
30.1.4.5. RECENT DEVELOPMENTS
30.1.5 ORGANON GROUP OF COMPANIES
30.1.5.1. COMPANY OVERVIEW
30.1.5.2. REVENUE ANALYSIS
30.1.5.3. GEOGRAPHIC PRESENCE
30.1.5.4. PRODUCT PORTFOLIO
30.1.5.5. RECENT DEVELOPMENTS
30.1.6 FRESENIUS KABI USA, LLC.
30.1.6.1. COMPANY OVERVIEW
30.1.6.2. REVENUE ANALYSIS
30.1.6.3. GEOGRAPHIC PRESENCE
30.1.6.4. PRODUCT PORTFOLIO
30.1.6.5. RECENT DEVELOPMENTS
30.1.7 CELLTRION USA INC.
30.1.7.1. COMPANY OVERVIEW
30.1.7.2. REVENUE ANALYSIS
30.1.7.3. GEOGRAPHIC PRESENCE
30.1.7.4. PRODUCT PORTFOLIO
30.1.7.5. RECENT DEVELOPMENTS
30.1.8 BIOCON BIOLOGICS INC.
30.1.8.1. COMPANY OVERVIEW
30.1.8.2. REVENUE ANALYSIS
30.1.8.3. GEOGRAPHIC PRESENCE
30.1.8.4. PRODUCT PORTFOLIO
30.1.8.5. RECENT DEVELOPMENTS
30.1.9 TEVA PHARMACEUTICALS, INC. + ALVOTECH USA INC.,
30.1.9.1. COMPANY OVERVIEW
30.1.9.2. REVENUE ANALYSIS
30.1.9.3. GEOGRAPHIC PRESENCE
30.1.9.4. PRODUCT PORTFOLIO
30.1.9.5. RECENT DEVELOPMENTS
30.1.10 COHERUS BIOSCIENCES INC.
30.1.10.1. COMPANY OVERVIEW
30.1.10.2. REVENUE ANALYSIS
30.1.10.3. GEOGRAPHIC PRESENCE
30.1.10.4. PRODUCT PORTFOLIO
30.1.10.5. RECENT DEVELOPMENTS
30.1.11 CATALENT INDIANA, LLC
30.1.11.1. COMPANY OVERVIEW
30.1.11.2. REVENUE ANALYSIS
30.1.11.3. GEOGRAPHIC PRESENCE
30.1.11.4. PRODUCT PORTFOLIO
30.1.11.5. RECENT DEVELOPMENTS
30.1.12 SAMSUNG BIOEPIS
30.1.12.1. COMPANY OVERVIEW
30.1.12.2. REVENUE ANALYSIS
30.1.12.3. GEOGRAPHIC PRESENCE
30.1.12.4. PRODUCT PORTFOLIO
30.1.12.5. RECENT DEVELOPMENTS
30.1.13 PFIZER INC.
30.1.13.1. COMPANY OVERVIEW
30.1.13.2. REVENUE ANALYSIS
30.1.13.3. GEOGRAPHIC PRESENCE
30.1.13.4. PRODUCT PORTFOLIO
30.1.13.5. RECENT DEVELOPMENTS
30.1.14 MYLAN N.V. AND FUJIFILM KYOWA KIRIN BIOLOGICS CO., LTD.
30.1.14.1. COMPANY OVERVIEW
30.1.14.2. REVENUE ANALYSIS
30.1.14.3. GEOGRAPHIC PRESENCE
30.1.14.4. PRODUCT PORTFOLIO
30.1.14.5. RECENT DEVELOPMENTS
30.1.15 INNOVENT BIOLOGICS, INC.
30.1.15.1. COMPANY OVERVIEW
30.1.15.2. REVENUE ANALYSIS
30.1.15.3. GEOGRAPHIC PRESENCE
30.1.15.4. PRODUCT PORTFOLIO
30.1.15.5. RECENT DEVELOPMENTS
30.1.16 RELIANCE LIFE SCIENCES
30.1.16.1. COMPANY OVERVIEW
30.1.16.2. REVENUE ANALYSIS
30.1.16.3. GEOGRAPHIC PRESENCE
30.1.16.4. PRODUCT PORTFOLIO
30.1.16.5. RECENT DEVELOPMENTS
30.1.17 HETERO
30.1.17.1. COMPANY OVERVIEW
30.1.17.2. REVENUE ANALYSIS
30.1.17.3. GEOGRAPHIC PRESENCE
30.1.17.4. PRODUCT PORTFOLIO
30.1.17.5. RECENT DEVELOPMENTS
30.1.18 TORRENT PHARMA
30.1.18.1. COMPANY OVERVIEW
30.1.18.2. REVENUE ANALYSIS
30.1.18.3. GEOGRAPHIC PRESENCE
30.1.18.4. PRODUCT PORTFOLIO
30.1.18.5. RECENT DEVELOPMENTS
30.1.19 SHANGHAI JUNSHI BIOSCIENCES CO LTD.
30.1.19.1. COMPANY OVERVIEW
30.1.19.2. REVENUE ANALYSIS
30.1.19.3. GEOGRAPHIC PRESENCE
30.1.19.4. PRODUCT PORTFOLIO
30.1.19.5. RECENT DEVELOPMENTS
30.1.20 ZYDUS LIFESCIENCES LTD.
30.1.20.1. COMPANY OVERVIEW
30.1.20.2. REVENUE ANALYSIS
30.1.20.3. GEOGRAPHIC PRESENCE
30.1.20.4. PRODUCT PORTFOLIO
30.1.20.5. RECENT DEVELOPMENTS
30.1.21 BIOXPRESS THERAPEUTICS SA
30.1.21.1. COMPANY OVERVIEW
30.1.21.2. REVENUE ANALYSIS
30.1.21.3. GEOGRAPHIC PRESENCE
30.1.21.4. PRODUCT PORTFOLIO
30.1.21.5. RECENT DEVELOPMENTS
30.1.22 BIO-THERA PHARMACEUTICALS CO., LTD.
30.1.22.1. COMPANY OVERVIEW
30.1.22.2. REVENUE ANALYSIS
30.1.22.3. GEOGRAPHIC PRESENCE
30.1.22.4. PRODUCT PORTFOLIO
30.1.22.5. RECENT DEVELOPMENTS
30.1.23 HISUN USA, INC.
30.1.23.1. COMPANY OVERVIEW
30.1.23.2. REVENUE ANALYSIS
30.1.23.3. GEOGRAPHIC PRESENCE
30.1.23.4. PRODUCT PORTFOLIO
30.1.23.5. RECENT DEVELOPMENTS
30.1.24 LG CHEM, LTD.
30.1.24.1. COMPANY OVERVIEW
30.1.24.2. REVENUE ANALYSIS
30.1.24.3. GEOGRAPHIC PRESENCE
30.1.24.4. PRODUCT PORTFOLIO
30.1.24.5. RECENT DEVELOPMENTS
30.2 PIPELINE
30.2.1 MOMENTA PHARMACEUTICALS, INC.
30.2.1.1. COMPANY OVERVIEW
30.2.1.2. REVENUE ANALYSIS
30.2.1.3. GEOGRAPHIC PRESENCE
30.2.1.4. PRODUCT PORTFOLIO
30.2.1.5. RECENT DEVELOPMENTS
30.2.2 MABXIENCE
30.2.2.1. COMPANY OVERVIEW
30.2.2.2. REVENUE ANALYSIS
30.2.2.3. GEOGRAPHIC PRESENCE
30.2.2.4. PRODUCT PORTFOLIO
30.2.2.5. RECENT DEVELOPMENTS
30.2.3 SHANGHAI HENLIUS BIOTECH, INC.
30.2.3.1. COMPANY OVERVIEW
30.2.3.2. REVENUE ANALYSIS
30.2.3.3. GEOGRAPHIC PRESENCE
30.2.3.4. PRODUCT PORTFOLIO
30.2.3.5. RECENT DEVELOPMENTS
30.2.4 BIOCAD
30.2.4.1. COMPANY OVERVIEW
30.2.4.2. REVENUE ANALYSIS
30.2.4.3. GEOGRAPHIC PRESENCE
30.2.4.4. PRODUCT PORTFOLIO
30.2.4.5. RECENT DEVELOPMENTS
30.2.5 PRESTIGE BIOPHARMA LTD.
30.2.5.1. COMPANY OVERVIEW
30.2.5.2. REVENUE ANALYSIS
30.2.5.3. GEOGRAPHIC PRESENCE
30.2.5.4. PRODUCT PORTFOLIO
30.2.5.5. RECENT DEVELOPMENTS
30.2.6 CINNAGEN CO.
30.2.6.1. COMPANY OVERVIEW
30.2.6.2. REVENUE ANALYSIS
30.2.6.3. GEOGRAPHIC PRESENCE
30.2.6.4. PRODUCT PORTFOLIO
30.2.6.5. RECENT DEVELOPMENTS
30.2.7 PLANTFORM CORPORATION
30.2.7.1. COMPANY OVERVIEW
30.2.7.2. REVENUE ANALYSIS
30.2.7.3. GEOGRAPHIC PRESENCE
30.2.7.4. PRODUCT PORTFOLIO
30.2.7.5. RECENT DEVELOPMENTS
30.2.8 OUTLOOK THERAPEUTICS, INC.
30.2.8.1. COMPANY OVERVIEW
30.2.8.2. REVENUE ANALYSIS
30.2.8.3. GEOGRAPHIC PRESENCE
30.2.8.4. PRODUCT PORTFOLIO
30.2.8.5. RECENT DEVELOPMENTS
30.2.9 NEUCLONE
30.2.9.1. COMPANY OVERVIEW
30.2.9.2. REVENUE ANALYSIS
30.2.9.3. GEOGRAPHIC PRESENCE
30.2.9.4. PRODUCT PORTFOLIO
30.2.9.5. RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
31 RELATED REPORTS
32 CONCLUSION
33 QUESTIONNAIRE
34 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.