Global Advance Anesthesia Monitoring Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
1.48 Billion
USD
3.21 Billion
2025
2033
USD
1.48 Billion
USD
3.21 Billion
2025
2033
| 2026 –2033 | |
| USD 1.48 Billion | |
| USD 3.21 Billion | |
|
|
|
|
Advance Anesthesia Monitoring Devices Market Size
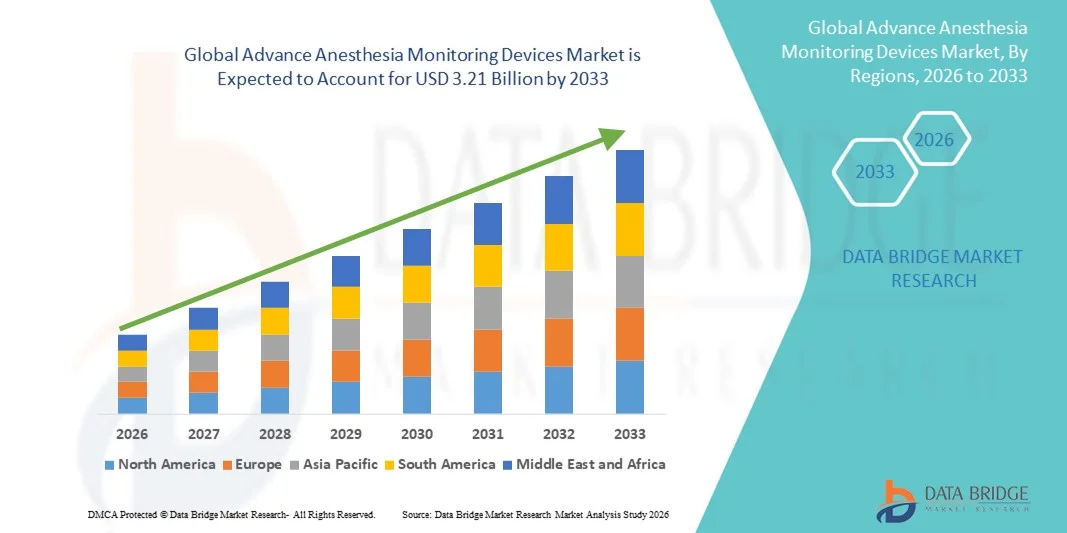
- The global advance anesthesia monitoring devices market size was valued at USD 1.48 billion in 2025 and is expected to reach USD 3.21 billion by 2033, at a CAGR of 10.19% during the forecast period
- The market growth is primarily driven by the rising volume of surgical procedures, increasing adoption of advanced patient monitoring technologies, and continuous technological advancements in anesthesia delivery and monitoring systems across hospitals and ambulatory surgical centers
- Furthermore, growing emphasis on patient safety, real-time monitoring of vital parameters, and demand for precise, integrated, and user-friendly anesthesia monitoring solutions are positioning advanced anesthesia monitoring devices as a critical component of modern perioperative care, thereby significantly supporting overall market expansion
Advance Anesthesia Monitoring Devices Market Analysis
- Advanced anesthesia monitoring devices, which support continuous and precise monitoring of patient physiological parameters during anesthesia administration, are becoming integral to modern surgical and perioperative environments due to their critical role in improving patient safety, clinical outcomes, and operational efficiency in hospitals and ambulatory surgical centers
- The increasing demand for advanced anesthesia monitoring devices is mainly driven by the rising number of surgical procedures globally, growing emphasis on anesthesia safety standards, and rapid technological advancements that enable more accurate, real-time monitoring and data integration
- North America dominated the advanced anesthesia monitoring devices market with the largest revenue share of 38.6% in 2025, supported by advanced healthcare infrastructure, high healthcare spending, and strong adoption of technologically sophisticated anesthesia monitoring solutions, with the U.S. leading due to high surgical volumes and stringent patient safety regulations
- Asia-Pacific is expected to be the fastest growing region in the advanced anesthesia monitoring devices market during the forecast period, attributed to improving healthcare infrastructure, increasing access to surgical care, rising medical tourism, and growing investments in advanced medical monitoring technologies
- Advanced anesthesia monitors segment dominated the advanced anesthesia monitoring devices market with a market share of 56.7% in 2025, driven by their enhanced functionality, integration of multiple monitoring parameters, and increasing preference among clinicians for comprehensive, real-time anesthesia monitoring systems that support improved perioperative decision-making
Report Scope and Advance Anesthesia Monitoring Devices Market Segmentation
|
Attributes |
Advance Anesthesia Monitoring Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Advance Anesthesia Monitoring Devices Market Trends
Integration of AI and Advanced Analytics for Precision Anesthesia Care
- A significant and accelerating trend in the global advanced anesthesia monitoring devices market is the integration of artificial intelligence (AI), advanced algorithms, and data analytics to support real-time clinical decision-making and personalized anesthesia management across surgical settings
- For instance, modern anesthesia monitoring systems increasingly incorporate AI-driven analytics to assess depth of anesthesia, predict hemodynamic instability, and alert clinicians to potential adverse events, enabling more proactive and precise intraoperative care
- AI-enabled anesthesia monitoring devices support features such as trend analysis of vital parameters, early warning systems for respiratory or cardiovascular compromise, and adaptive monitoring based on patient-specific responses, thereby improving overall perioperative safety and efficiency
- The integration of advanced anesthesia monitors with hospital information systems and electronic health records enables centralized data visualization, seamless documentation, and improved coordination among anesthesiologists, surgeons, and perioperative care teams
- This trend toward intelligent, connected, and data-driven anesthesia monitoring solutions is reshaping clinical expectations for patient safety and workflow optimization, prompting companies to develop advanced monitors with enhanced processing capabilities and interoperability
- The demand for advanced anesthesia monitoring devices with AI-driven insights and real-time analytics is rising steadily across hospitals and ambulatory surgical centers, as healthcare providers prioritize accuracy, safety, and efficiency in anesthesia delivery
- Increasing focus on minimally invasive and outpatient surgeries is driving demand for compact, user-friendly advanced anesthesia monitors that support rapid setup and high patient turnover
Advance Anesthesia Monitoring Devices Market Dynamics
Driver
Rising Surgical Volumes and Emphasis on Patient Safety
- The increasing volume of surgical procedures worldwide, combined with a strong emphasis on improving patient safety and anesthesia outcomes, is a major driver fueling demand for advanced anesthesia monitoring devices
- For instance, hospitals and surgical centers are increasingly adopting advanced anesthesia monitors to comply with evolving safety guidelines and reduce the risk of anesthesia-related complications during complex procedures
- As healthcare providers focus on minimizing perioperative risks, advanced anesthesia monitoring devices offer continuous, real-time assessment of vital parameters such as oxygenation, ventilation, and hemodynamic status, representing a significant upgrade over basic monitoring tools
- Furthermore, growing awareness among clinicians regarding the benefits of precise anesthesia monitoring, including reduced adverse events and improved recovery outcomes, is accelerating adoption across both developed and emerging healthcare markets
- The expansion of ambulatory surgical centers and the shift toward minimally invasive surgeries are further driving demand for compact, integrated, and reliable anesthesia monitoring solutions that support high patient throughput and clinical efficiency
- Increasing geriatric population globally, which is more susceptible to surgical and anesthesia-related risks, is strengthening the need for advanced and continuous anesthesia monitoring solutions
- Rising healthcare investments in developing regions to modernize operating rooms and perioperative care infrastructure are further supporting market growth
Restraint/Challenge
High Device Costs and Regulatory Compliance Complexity
- The high cost associated with advanced anesthesia monitoring devices, along with complex regulatory approval requirements, poses a notable challenge to widespread adoption, particularly in resource-constrained healthcare settings
- For instance, stringent regulatory standards governing medical device safety, performance, and clinical validation can prolong product approval timelines and increase development costs for manufacturers
- Compliance with diverse regulatory frameworks across regions requires significant investment in testing, certification, and documentation, which can limit the speed of innovation and market entry for new advanced monitoring technologies
- In addition, the relatively high upfront investment required for advanced anesthesia monitors compared to basic monitoring systems can deter adoption among smaller hospitals and surgical centers with limited capital budgets
- Limited availability of trained anesthesiology professionals capable of fully utilizing advanced monitoring features can restrict optimal device adoption in some healthcare facilities
- Interoperability challenges between advanced anesthesia monitors and legacy hospital IT systems may increase integration complexity and implementation costs
- Overcoming these challenges through cost optimization, streamlined regulatory pathways, training initiatives, and improved system compatibility will be essential for sustained market expansion
Advance Anesthesia Monitoring Devices Market Scope
The market is segmented on the basis of product type and end user.
- By Product Type
On the basis of product type, the advanced anesthesia monitoring devices market is segmented into basic anesthesia monitors, advanced anesthesia monitors, and integrated anesthesia workstations. The advanced anesthesia monitors segment dominated the market with the largest market revenue share of 56.7% in 2025, driven by their ability to provide continuous, real-time monitoring of multiple critical parameters such as oxygenation, ventilation, and hemodynamics during surgical procedures. Healthcare providers increasingly prefer advanced monitors due to their higher accuracy, enhanced patient safety features, and support for complex and high-risk surgeries. These devices are widely adopted in tertiary hospitals and specialty surgical centers where precision anesthesia management is essential. The integration of alarms, data analytics, and connectivity with hospital systems further strengthens their dominance. In addition, growing regulatory emphasis on anesthesia safety standards supports higher adoption of advanced monitoring solutions over basic systems.
The integrated anesthesia workstations segment is expected to be the fastest growing from 2026 to 2033, fueled by the increasing demand for all-in-one solutions that combine anesthesia delivery, ventilation, and advanced monitoring in a single platform. Integrated workstations improve workflow efficiency, reduce equipment footprint in operating rooms, and minimize setup complexity for clinicians. Hospitals upgrading or expanding surgical infrastructure increasingly favor these systems to streamline perioperative care. The rising number of complex and minimally invasive surgeries further supports adoption, as integrated workstations enable seamless coordination between anesthesia administration and monitoring. Technological advancements and growing investments in modern operating room setups are accelerating the growth of this segment.
- By End User
On the basis of end user, the advanced anesthesia monitoring devices market is segmented into hospitals, private clinics, ambulatory services, and others. The hospitals segment dominated the market with the largest revenue share in 2025, driven by high surgical volumes, availability of advanced infrastructure, and greater capacity to invest in sophisticated anesthesia monitoring technologies. Hospitals handle a wide range of major and complex surgical procedures that require continuous and advanced anesthesia monitoring to ensure patient safety. The presence of trained anesthesiologists and compliance with strict regulatory and clinical safety standards further supports strong adoption in hospital settings. Large public and private hospitals also tend to replace or upgrade legacy systems more frequently, contributing to sustained demand.
The ambulatory services segment is projected to witness the fastest growth during the forecast period, supported by the rapid expansion of ambulatory surgical centers and the shift toward outpatient and minimally invasive procedures. These facilities require compact, efficient, and reliable anesthesia monitoring devices that support quick patient turnover and high procedural efficiency. Advances in portable and user-friendly monitoring systems make them well suited for ambulatory settings. Cost-effectiveness, reduced hospital stays, and patient preference for outpatient procedures are accelerating the growth of ambulatory services. As a result, demand for advanced anesthesia monitoring devices tailored to ambulatory care environments is rising steadily.
Advance Anesthesia Monitoring Devices Market Regional Analysis
- North America dominated the advanced anesthesia monitoring devices market with the largest revenue share of 38.6% in 2025, supported by advanced healthcare infrastructure, high healthcare spending, and strong adoption of technologically sophisticated anesthesia monitoring solutions, with the U.S. leading due to high surgical volumes and stringent patient safety regulations
- Healthcare providers in the region place significant value on real-time, high-precision anesthesia monitoring solutions that support improved clinical outcomes, regulatory compliance, and efficient operating room workflows
- This widespread adoption is further supported by high healthcare expenditure, rapid uptake of advanced medical technologies, and the strong presence of leading medical device manufacturers, establishing advanced anesthesia monitoring devices as a standard component across hospitals and ambulatory surgical centers
U.S. Advance Anesthesia Monitoring Devices Market Insight
The U.S. advanced anesthesia monitoring devices market captured the largest revenue share of 72% in 2025 within North America, fueled by high surgical volumes, advanced healthcare infrastructure, and stringent patient safety regulations. Hospitals and ambulatory surgical centers increasingly prioritize precise, real-time monitoring solutions to enhance perioperative outcomes. The growing adoption of integrated anesthesia workstations and AI-enabled monitors, alongside rising investments in modern operating room technologies, is further propelling market growth. Moreover, the expansion of minimally invasive surgeries and outpatient procedures is significantly contributing to the demand for advanced monitoring systems.
Europe Advanced Anesthesia Monitoring Devices Market Insight
The Europe advanced anesthesia monitoring devices market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by regulatory mandates emphasizing patient safety and the growing demand for technologically advanced monitoring solutions in hospitals. Increasing urbanization, coupled with rising healthcare expenditure, is fostering the adoption of advanced monitors. European healthcare providers are also emphasizing enhanced surgical outcomes and reduced anesthesia-related complications. The region is witnessing significant growth across hospitals, specialty surgical centers, and ambulatory care facilities, with advanced monitors being incorporated into both new setups and upgraded operating rooms.
U.K. Advanced Anesthesia Monitoring Devices Market Insight
The U.K. advanced anesthesia monitoring devices market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the increasing focus on perioperative safety and quality of care. Concerns regarding anesthesia-related complications are encouraging hospitals and surgical centers to adopt advanced monitoring solutions. In addition, the U.K.’s emphasis on modern healthcare infrastructure and digital integration in operating rooms is expected to continue stimulating market growth. Growing adoption of AI-assisted anesthesia systems and integration with electronic health records further supports this trend.
Germany Advanced Anesthesia Monitoring Devices Market Insight
The Germany advanced anesthesia monitoring devices market is expected to expand at a considerable CAGR during the forecast period, fueled by a strong healthcare system, high surgical volumes, and rising investments in technologically advanced perioperative care solutions. Hospitals increasingly adopt integrated anesthesia workstations and advanced monitors to improve patient safety, workflow efficiency, and surgical outcomes. The country’s focus on innovation, precision medicine, and compliance with strict regulatory standards promotes the adoption of these devices. Growing awareness among healthcare providers about real-time anesthesia monitoring benefits is also driving growth.
Asia-Pacific Advanced Anesthesia Monitoring Devices Market Insight
The Asia-Pacific advanced anesthesia monitoring devices market is poised to grow at the fastest CAGR of 23% during the forecast period of 2026 to 2033, driven by expanding healthcare infrastructure, rising surgical volumes, and increased focus on patient safety in countries such as China, Japan, and India. Government initiatives to modernize hospitals and promote digital health technologies are fueling the adoption of advanced monitoring systems. In addition, the region’s rising medical tourism, expanding ambulatory surgical centers, and increasing availability of cost-effective advanced monitors are significantly contributing to market growth.
Japan Advanced Anesthesia Monitoring Devices Market Insight
The Japan advanced anesthesia monitoring devices market is gaining momentum due to high surgical procedure volumes, technological advancements in perioperative care, and emphasis on patient safety. Hospitals and clinics increasingly adopt AI-enabled and multi-parameter anesthesia monitors to improve real-time decision-making and surgical outcomes. Integration of these devices with hospital IT systems, electronic health records, and other perioperative equipment is fueling growth. Moreover, the aging population and rising outpatient procedures are driving demand for efficient, user-friendly monitoring solutions in both hospital and ambulatory care settings.
India Advanced Anesthesia Monitoring Devices Market Insight
The India advanced anesthesia monitoring devices market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to rapid urbanization, growing surgical volumes, and expanding healthcare infrastructure. India is witnessing rising adoption of advanced anesthesia monitors in hospitals, private clinics, and ambulatory surgical centers. Government initiatives toward modernizing perioperative care, coupled with increasing investments in smart operating rooms, are key factors propelling market growth. The availability of cost-effective solutions and increasing awareness among clinicians regarding patient safety benefits are further driving demand.
Advance Anesthesia Monitoring Devices Market Share
The Advance Anesthesia Monitoring Devices industry is primarily led by well-established companies, including:
- GE HealthCare (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- Medtronic (Ireland)
- Drägerwerk AG & Co. KGaA (Germany)
- Masimo Corporation (U.S.)
- Mindray Medical International Limited (China)
- NIHON KOHDEN CORPORATION (Japan)
- Spacelabs Healthcare (U.S.)
- BD (U.S.)
- Infinium Medical, Inc. (U.S.)
- Schiller AG (Switzerland)
- HEYER Medical AG (Germany)
- Siare Engineering International Group S.r.l. (Italy)
- ICU Medical, Inc. (U.S.)
- B. Braun SE (Germany)
- Teleflex Incorporated (U.S.)
- Fisher & Paykel Healthcare Limited (New Zealand)
- Penlon Ltd (U.K.)
- Nonin Medical, Inc. (U.S.)
- Beijing Aeonmed Co., Ltd. (China)
What are the Recent Developments in Global Advance Anesthesia Monitoring Devices Market?
- In October 2025, GE HealthCare unveiled the Carestation 850, its next-generation anesthesia delivery system with advanced tools, customizable applications, and enhanced user interfaces. The system is designed to address evolving clinical needs, support workflow efficiency, and improve perioperative performance for clinicians, pending FDA 510(k) clearance following CE and Australian approvals
- In October 2025, Philips and Getinge announced a commercial partnership to deliver integrated anesthesia and monitoring solutions. The collaboration combines Getinge’s anesthesia delivery systems with Philips’ IntelliVue monitoring technology, offering hospitals a unified anesthesia workstation with seamless data flow and simplified operation
- In August 2025, Dräger’s Atlan A350/A350 XL anesthesia workstations received Authority to Operate (ATO) certification under the U.S. Department of Defense’s Risk Management Framework, marking a major cybersecurity milestone for anesthesia equipment used in hospitals and critical care environments by ensuring the devices meet rigorous federal standards for protecting patient data and operational integrity while supporting complex surgical procedures
- In March 2024, Medtronic announced that the BIS™ Advance monitor received U.S. FDA 510(k) clearance, enabling anesthesia providers to personalize anesthesia dosing based on brain activity. The monitor uses the clinically validated BIS™ algorithm with a redesigned interface to help clinicians tailor anesthetic depth, potentially improving recovery outcomes and reducing anesthetic use
- In September 2023, Dräger launched the new Atlan anesthesia workstation family in the United States, offering a comprehensive anesthesia platform with enhanced safety functions, precision ventilation, and customizable configurations suitable for all patient age groups, from neonates to adults, significantly expanding clinical options for perioperative monitoring and support in operating rooms
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.