Global Aids Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
35.33 Billion
USD
47.61 Billion
2024
2032
USD
35.33 Billion
USD
47.61 Billion
2024
2032
| 2025 –2032 | |
| USD 35.33 Billion | |
| USD 47.61 Billion | |
|
|
|
|
Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Size
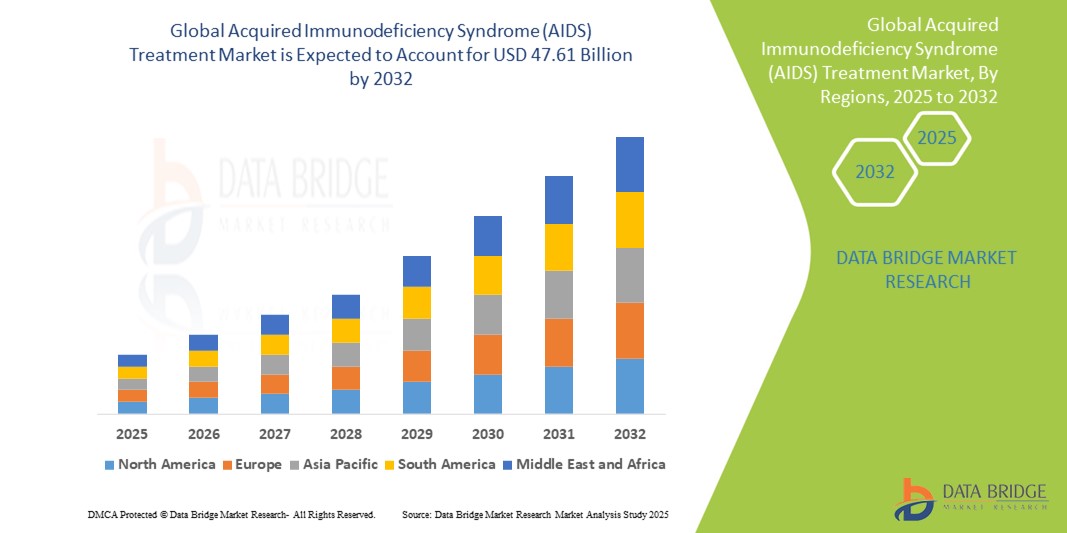
- The global acquired immunodeficiency syndrome (AIDS) treatment market size was valued at USD 35.33 billion in 2024 and is expected to reach USD 47.61 billion by 2032, at a CAGR of 3.80% during the forecast period
- The market growth is primarily driven by the increasing global prevalence of HIV infections, combined with improved access to antiretroviral therapy (ART) in both developed and developing regions
- In addition, continuous advancements in treatment regimens, including long-acting injectables and novel combination therapies, are transforming patient outcomes and compliance, thereby strengthening market expansion. These medical and public health developments are significantly accelerating the global adoption of effective AIDS treatment solutions, fueling industry growth
Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Analysis
- AIDS treatment, centered around antiretroviral therapy (ART), is an essential component of global healthcare aimed at managing HIV infection, preventing disease progression, and reducing transmission through sustained viral suppression across both developed and developing healthcare settings
- The rising demand for AIDS treatment is primarily driven by the increasing global prevalence of HIV, expanding public health initiatives, and improved access to affordable and effective ART regimens supported by international health organizations and government programs
- North America dominated the acquired immunodeficiency syndrome (AIDS) treatment market with the largest revenue share of 42.3% in 2024, characterized by advanced healthcare systems, early adoption of innovative treatment options, high awareness levels, and strong pharmaceutical R&D pipelines, particularly in the U.S., which continues to lead in ART development and deployment
- Asia-Pacific is expected to be the fastest growing region in the acquired immunodeficiency syndrome (AIDS) treatment market during the forecast period due to increasing HIV incidence, rising healthcare expenditures, and expanded access to ART programs supported by regional governments and international collaborations
- Nucleoside/nucleotide reverse transcriptase inhibitors segment dominated the acquired immunodeficiency syndrome (AIDS) treatment market with a market share of 40.1% in 2024, driven by its proven efficacy, inclusion in first-line treatment guidelines, and widespread global usage
Report Scope and Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Segmentation
|
Attributes |
Acquired Immunodeficiency Syndrome (AIDS) Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Trends
“Advancements in Long-Acting Therapies and Personalized Treatment”
- A significant and accelerating trend in the global AIDS treatment market is the growing shift toward long-acting antiretroviral therapies (LA-ART) and personalized treatment approaches, which are substantially improving patient adherence, reducing dosing frequency, and enhancing quality of life
- For instance, long-acting injectable therapies such as Cabotegravir and Rilpivirine are now available in multiple regions, offering monthly or bi-monthly treatment options that eliminate the need for daily oral intake. This innovation reduces stigma and simplifies treatment routines, especially for younger or mobile populations
- Advancements in personalized medicine allow healthcare providers to tailor antiretroviral regimens based on viral genotyping and patient-specific resistance profiles. This enables the selection of the most effective drug combinations, thereby minimizing side effects and improving long-term outcomes
- Further innovations include the development of extended-release implants and subcutaneous drug delivery systems, designed to maintain therapeutic drug levels for months with a single administration. These technologies are particularly promising for resource-limited settings with constrained healthcare access
- The integration of digital tools such as mobile adherence apps, smart pill dispensers, and remote monitoring systems is enhancing care continuity and enabling early intervention in case of non-adherence or adverse reactions. These tech-enabled solutions are becoming an important adjunct to traditional HIV care
- This trend toward long-acting, individualized, and tech-supported treatment regimens is transforming how AIDS is managed across global healthcare systems. Companies such as Gilead Sciences and ViiV Healthcare are leading the charge, developing next-generation therapies that align with patient preferences and clinical best practices
- The demand for simplified, effective, and patient-friendly AIDS treatment solutions is rising steadily across both developed and emerging markets, driven by the need for improved adherence, reduced stigma, and more flexible treatment options
Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Dynamics
Driver
“Rising Global HIV Prevalence and Expanding Access to Antiretroviral Therapy (ART)”
- The growing global burden of HIV infections, particularly in low- and middle-income countries, coupled with expanded access to life-saving antiretroviral therapies, is a major driver fueling the demand for AIDS treatment solutions
- For instance, in January 2024, ViiV Healthcare announced the expansion of access to its long-acting injectable treatment, Cabenuva, in partnership with global health organizations to reach underserved regions in Africa and Southeast Asia. Such initiatives by key players are expected to accelerate ART uptake during the forecast period
- As awareness campaigns, screening programs, and international funding from organizations such as UNAIDS and the Global Fund continue to scale, more individuals are being diagnosed and linked to care, creating consistent demand for affordable, effective treatment options
- Furthermore, policy support from governments and the integration of HIV treatment into national healthcare programs are boosting ART availability, particularly through generic manufacturing and fixed-dose combinations that simplify therapy and improve adherence
- The push for universal healthcare access, combined with advancements in treatment delivery such as long-acting injectables, mobile clinics, and decentralized treatment models, is improving ART coverage in both urban and rural settings, contributing significantly to the overall growth of the AIDS treatment market
Restraint/Challenge
“Side Effects of ART and Regulatory & Accessibility Barriers”
- Despite the effectiveness of antiretroviral therapy (ART), treatment-related side effects such as nausea, fatigue, lipodystrophy, and long-term organ complications remain significant challenges, often leading to poor adherence and therapy discontinuation among patients
- For instance, some older-generation ART drugs have been associated with adverse effects on kidney and bone health, limiting their long-term use and necessitating careful monitoring, particularly in aging HIV-positive populations
- Managing these side effects through newer, better-tolerated regimens is essential for improving adherence and overall treatment outcomes. Companies such as Gilead Sciences and ViiV Healthcare continue to invest in next-generation formulations with reduced toxicity profiles, yet cost remains a barrier in many settings
- Moreover, navigating complex regulatory pathways for the approval and distribution of new ART drugs, especially in low-income countries, slows the pace of access and innovation. Licensing restrictions, limited local infrastructure, and fragmented procurement systems can delay the introduction of advanced therapies
- In addition, healthcare disparities and stigma continue to prevent many individuals from seeking timely diagnosis and treatment, particularly in rural or socially marginalized populations. While progress has been made through global initiatives, these systemic challenges still limit the effectiveness of comprehensive HIV/AIDS management
- Overcoming these restraints requires continued investment in safer ART options, harmonized regulatory frameworks, expanded global health funding, and community-based outreach programs to reduce stigma and improve access, especially in high-burden regions
Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Scope
The market is segmented on the basis of type, mechanism of action, drugs, route of administration, distribution channel, and end users.
- By Type
On the basis of type, the acquired immunodeficiency syndrome (AIDS) treatment market is segmented into HIV-1 and HIV-2. The HIV-1 segment dominated the market in 2024, accounting for the largest revenue share, owing to its significantly higher global prevalence and more aggressive disease progression. HIV-1 is the most common and pathogenic strain worldwide, particularly in North America, Europe, and sub-Saharan Africa, which drives demand for its specific treatment regimens and ongoing drug development.
The HIV-2 segment, while less prevalent and largely confined to West Africa, is projected to see fastest growth during forecast period, due to improving diagnostic capabilities and increased global awareness of non-HIV-1 infections.
- By Mechanism Of Action
On the basis of mechanism of action, the acquired immunodeficiency syndrome (AIDS) treatment market is categorized into nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), fusion inhibitors, CCR5 antagonist, integrase inhibitors, monoclonal antibody, and others. The nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) segment led the market with a market share of 40.1% in 2024 with the largest revenue share due to its historical significance as a cornerstone of ART and inclusion in most first-line regimens recommended by global health organizations. These drugs are well-studied, widely accessible, and available in affordable generic versions.
The Integrase Inhibitors segment is expected to witness the fastest growth from 2025 to 2032, attributed to its superior efficacy, fewer side effects, and increasing preference for inclusion in modern first-line therapies, particularly in high-income countries.
- By Drugs
On the basis of drugs, the acquired immunodeficiency syndrome (AIDS) treatment market is segmented into abacavir, delavirdine, atazanavir, enfuvirtide, maraviroc, dolutegravir, ibalizumab, and others. Dolutegravir held the largest market share in 2024, recognized for its high barrier to resistance, favorable safety profile, and widespread inclusion in national treatment guidelines. Its use in both first-line and second-line regimens has contributed to its global dominance.
Ibalizumab, a monoclonal antibody, is projected to witness the highest CAGR during the forecast period due to its role in treating multidrug-resistant HIV cases and its novel mechanism, making it vital in salvage therapy protocols.
- By Route Of Administration
On the basis of route of administration, the acquired immunodeficiency syndrome (AIDS) treatment market is segmented into oral, intravenous, and others. The oral route dominated the market in 2024, with the highest market revenue share, driven by the convenience, cost-effectiveness, and adherence advantages associated with daily pills and fixed-dose combinations.
The Intravenous segment is expected to grow rapidly during the forecast period, supported by the growing uptake of long-acting injectable ARTs, particularly in high-income markets where monthly or bi-monthly treatments are preferred for convenience and reduced stigma.
- By Distribution Channel
On the basis of distribution channel, the acquired immunodeficiency syndrome (AIDS) treatment market is divided into direct, online pharmacy, retailers, and others. Direct distribution held the largest revenue share in 2024, primarily due to bulk procurement by governments, international health organizations, and hospitals under structured ART delivery programs.
The Online Pharmacy segment is expected to grow at the fastest rate during forecast period, fueled by the increasing digitalization of healthcare access, telemedicine integration, and growing demand for discreet, home-delivered treatment solutions.
- By End Users
On the basis of end users, the acquired immunodeficiency syndrome (AIDS) treatment market is segmented into hospitals, homecare, specialty clinics, and others. Hospitals dominated the market in 2024, owing to the centralized distribution of ART, availability of diagnostic and monitoring facilities, and the critical care required during opportunistic infections.
The Homecare segment is projected to witness the fastest growth from 2025 to 2032, as long-acting therapies and digital adherence tools make decentralized treatment and at-home care more viable, especially for stable patients seeking flexibility and privacy.
Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Regional Analysis
- North America dominated the acquired immunodeficiency syndrome (AIDS) treatment market with the largest revenue share of 42.3% in 2024, driven by advanced healthcare systems, early adoption of innovative treatment options, high awareness levels, and strong pharmaceutical R&D pipelines
- Patients in the region benefit from widespread access to ART, strong government support programs, and robust insurance coverage, which together facilitate early diagnosis and sustained treatment adherence
- The region’s leadership is further supported by significant investments in HIV research, the presence of leading pharmaceutical companies, and the availability of cutting-edge treatment options such as long-acting injectables and personalized regimens, positioning North America as a hub for ongoing innovation and effective HIV/AIDS management
U.S. Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Insight
The U.S. acquired immunodeficiency syndrome (AIDS) treatment market captured the largest revenue share of 75.2% in 2024 within North America, driven by high awareness, early diagnosis, and widespread access to advanced antiretroviral therapies (ART). Strong federal support programs such as the Ryan White HIV/AIDS Program and a robust network of healthcare providers ensure continuity of care. In addition, the U.S. leads in ART innovation, with pharmaceutical companies actively developing and launching next-generation therapies such as long-acting injectables and personalized treatment regimens.
Europe Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Insight
The Europe acquired immunodeficiency syndrome (AIDS) treatment market is projected to expand at a steady CAGR throughout the forecast period, supported by strong healthcare infrastructure, favorable reimbursement policies, and increasing government initiatives to end the HIV epidemic. Rising public health awareness and routine screening are improving early diagnosis and treatment rates. The market is also benefiting from growing adoption of novel ART options and increasing access to HIV care among key populations, particularly in countries such as France, Spain, and the Netherlands.
U.K. Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Insight
The U.K. acquired immunodeficiency syndrome (AIDS) treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by NHS-backed HIV prevention and treatment programs and improved access to pre-exposure prophylaxis (PrEP). The government’s ambitious target to eliminate new HIV transmissions by 2030 is driving large-scale testing and treatment coverage. In addition, the increasing availability of innovative ART and long-acting regimens enhances patient convenience and adherence, fueling market expansion
Germany Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Insight
The Germany acquired immunodeficiency syndrome (AIDS) treatment market is expected to expand at a considerable CAGR during the forecast period, driven by a high standard of care, government-backed prevention initiatives, and a strong pharmaceutical presence. Germany’s structured HIV care programs and integration of ART in national health plans ensure broad access. Emphasis on patient-centered care, including individualized regimens and improved drug tolerability, supports continued uptake of advanced treatment options.
Asia-Pacific Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Insight
The Asia-Pacific acquired immunodeficiency syndrome (AIDS) treatment market is poised to grow at the fastest CAGR during 2025 to 2032, driven by increasing HIV prevalence, growing public health efforts, and expanded access to ART in countries such as China, India, and Thailand. The region’s improving healthcare infrastructure, combined with supportive government initiatives and international funding, is accelerating diagnosis and treatment. In addition, increasing generic drug production in APAC countries is enhancing affordability and accessibility.
Japan Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Insight
The Japan acquired immunodeficiency syndrome (AIDS) treatment market is gaining momentum due to strong government involvement, early adoption of advanced ART, and an emphasis on public health education. Widespread screening, along with comprehensive national health insurance coverage, ensures high treatment accessibility. Japan’s focus on innovation, aging population, and integration of telemedicine in HIV care are further supporting the demand for effective, patient-friendly treatment options.
India Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Insight
The India acquired immunodeficiency syndrome (AIDS) treatment market accounted for the largest market revenue share in Asia Pacific in 2024, supported by large-scale government initiatives such as NACO and expanded ART availability across public health facilities. Rapid urbanization, increased awareness, and the scaling up of HIV testing and treatment services are driving market growth. India’s role as a leading producer of affordable generic ART drugs also boosts access across rural and underserved regions, solidifying its position as a key regional market.
Acquired Immunodeficiency Syndrome (AIDS) Treatment Market Share
The acquired immunodeficiency syndrome (AIDS) treatment industry is primarily led by well-established companies, including:
- Gilead Sciences, Inc. (U.S.)
- ViiV Healthcare group (U.K.)
- Johnson & Johnson Services, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- AbbVie Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Theratechnologies Inc. (Canada)
- Cipla Ltd. (India)
- Hetero Labs Limited (India)
- Aurobindo Pharma Limited (India)
- GSK plc (U.K.)
- Sun Pharmaceutical Industries Ltd. (India)
- Dr. Reddy's Laboratories Ltd. (India)
- Boehringer Ingelheim International GmbH (Germany)
- Pfizer Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Shanghai Fosun Pharmaceutical (Group) Co., Ltd. (China)
- MacroGenics, Inc. (U.S.)
- Emcure Pharmaceuticals Ltd. (India)
What are the Recent Developments in Global Acquired Immunodeficiency Syndrome (AIDS) Treatment Market?
- In March 2024, ViiV Healthcare, a specialist HIV company majority-owned by GSK, received expanded FDA approval for its long-acting injectable regimen Cabenuva (cabotegravir and rilpivirine) for broader use in adolescent patients aged 12 and older. This development marks a significant step forward in making simplified, low-frequency dosing options accessible to younger populations, enhancing adherence and reducing stigma, particularly in high-burden settings
- In February 2024, Gilead Sciences Inc. initiated Phase III trials for a novel once-weekly oral antiretroviral therapy combining lenacapavir with investigational agents, targeting better adherence and reduced resistance. The advancement reinforces Gilead's leadership in developing long-acting HIV treatments and represents a shift toward more patient-centric regimens
- In January 2024, the Joint United Nations Programme on HIV/AIDS (UNAIDS) launched a multi-country initiative focused on eliminating vertical transmission of HIV in sub-Saharan Africa by 2030. The program, supported by new treatment guidelines and ART scale-up strategies, emphasizes the importance of early maternal ART intervention and continuous treatment access, signaling intensified global commitment to eradicating AIDS among children
- In December 2023, Cipla Limited partnered with the Clinton Health Access Initiative (CHAI) to expand the distribution of affordable, high-quality antiretroviral drugs in low-income countries. This collaboration aims to improve accessibility by strengthening supply chains and facilitating generic ART production under voluntary licensing agreements, particularly in Asia and Africa
- In November 2023, Theratechnologies Inc. announced positive trial results for its investigational therapy TH1902, a peptide-drug conjugate targeting HIV-infected cells with enhanced precision. This innovative approach offers potential to overcome traditional ART limitations, reduce viral reservoirs, and address long-term treatment goals. The progress reflects ongoing innovation in next-generation AIDS therapies aimed at improving outcomes and quality of life
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.