Global Alad Porphyria Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
500.50 Million
USD
803.76 Million
2025
2033
USD
500.50 Million
USD
803.76 Million
2025
2033
| 2026 –2033 | |
| USD 500.50 Million | |
| USD 803.76 Million | |
|
|
|
|
ALAD Porphyria Treatment Market Size
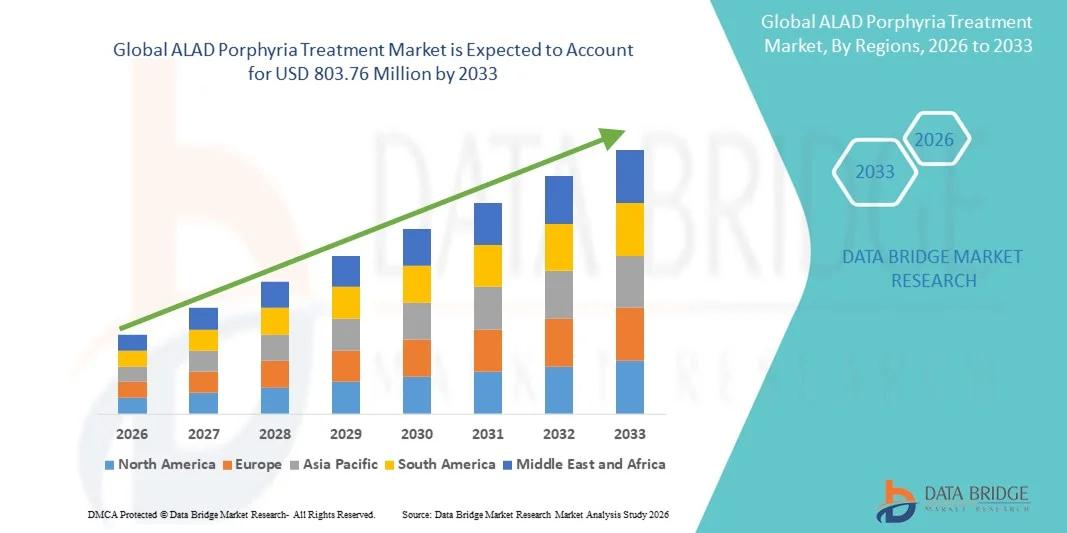
- The global ALAD porphyria treatment market size was valued at USD 500.50 Million in 2025 and is expected to reach USD 803.76 Million by 2033, at a CAGR of 6.10% during the forecast period
- The market growth is largely fueled by rising awareness of porphyria disorders, advancements in diagnostic technologies, and increasing availability of targeted therapeutic options for ALAD Porphyria. Growing clinical research activities and improved understanding of the genetic basis of the disease are contributing to earlier diagnosis and better treatment outcomes, thereby driving adoption across healthcare settings
- Furthermore, the surge in R&D investments, development of novel enzyme-replacement therapies, and supportive regulatory initiatives for rare disease treatments are significantly boosting the demand for ALAD Porphyria Treatment solutions. These converging factors are accelerating the uptake of innovative therapies and care approaches, thereby substantially strengthening the overall industry growth
ALAD Porphyria Treatment Market Analysis
- ALAD Porphyria Treatment, which focuses on managing delta-aminolevulinic acid dehydratase deficiency through targeted therapies and supportive care, is becoming increasingly important in rare disease management due to growing clinical awareness, improved diagnostic capabilities, and advancements in genetic testing
- The escalating demand for ALAD porphyria treatment solutions is primarily fueled by increasing research initiatives, a rise in diagnosed patient populations, expanding access to specialized treatment centers, and growing emphasis on the development of novel therapies for ultra-rare metabolic disorders
- North America dominated the ALAD porphyria treatment market with the largest revenue share of 40.00% in 2025, characterized by strong research infrastructure, high healthcare spending, and the presence of leading biopharmaceutical companies, with the U.S. experiencing substantial growth in clinical trials, patient registries, and adoption of advanced therapeutic interventions driven by innovations in genetic and enzyme-based therapies
- Asia-Pacific is expected to be the fastest-growing region in the ALAD Porphyria Treatment market during the forecast period due to increasing healthcare modernization, rising investments in rare disease diagnosis, and expanding awareness programs led by medical organizations and government bodies
- . Molecular genetic testing dominated the largest market revenue share of 52.3% in 2025, driven by its accuracy in detecting genetic mutations responsible for ALAD Porphyria. Clinicians increasingly rely on genetic confirmation for precise diagnosis
Report Scope and ALAD Porphyria Treatment Market Segmentation
|
Attributes |
ALAD Porphyria Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
ALAD Porphyria Treatment Market Trends
Advancing Treatment Through Precision Medicine and Novel Therapeutic Research
- A significant and accelerating trend in the global ALAD porphyria treatment market is the growing shift toward precision medicine, biomarker-based diagnosis, and targeted therapeutic approaches. This transition is improving accuracy in disease identification and enabling more individualized treatment pathways for patients
- For instance, several research groups from the U.S. and Europe are actively developing genetic and biochemical assays that detect ALAD enzyme deficiencies with greater sensitivity, supporting earlier and more precise intervention
- Advancements in drug delivery technologies, such as sustained-release formulations and biologics designed to enhance heme biosynthesis, are reshaping the treatment landscape. These innovations help optimize therapeutic outcomes and reduce the frequency of clinical episodes
- The integration of digital health monitoring tools—such as portable biochemical analyzers and symptom-tracking applications—is enabling clinicians to remotely monitor disease fluctuations and personalize therapy plans more effectively
- Furthermore, ongoing research into gene therapy, enzyme-replacement models, and RNA-based approaches is opening new avenues for long-term disease modification. Several academic institutions and biotech companies are running exploratory preclinical studies to restore heme pathway stability
- This trend toward more advanced, stable, and personalized treatment models is significantly transforming clinical expectations. As a result, companies and research organizations are accelerating trials focused on novel therapeutics and next-generation diagnostic system
- The demand for innovative, evidence-based, and targeted ALAD Porphyria Treatment solutions continues to rise as healthcare systems prioritize early diagnosis, improved management protocols, and long-term disease stabilization
ALAD Porphyria Treatment Market Dynamics
Driver
Increasing Need Due to Rising Metabolic Disorders and Improved Diagnostic Capabilities
- The growing prevalence of metabolic disorders, hereditary porphyria conditions, and enzyme-deficiency-related complications is a major driver accelerating demand for ALAD Porphyria Treatment worldwide
- For instance, in February 2024, researchers at the Karolinska Institute reported progress in developing advanced biomarkers that can detect early-stage ALAD enzyme dysfunction, supporting faster diagnosis and intervention
- As awareness rises among clinicians and patients, early detection programs are expanding, enabling timely treatment initiation and improved clinical outcomes
- In addition, advancements in laboratory infrastructure, including high-performance liquid chromatography (HPLC) and mass spectrometry systems, have boosted diagnostic accuracy, encouraging wider screening adoption
- The increasing adoption of supportive therapies such as heme infusions, pain-management protocols, and metabolic stabilizers is further strengthening treatment demand across hospitals, specialty clinics, and research centers
- Moreover, the expansion of rare disease research funding and inclusion of porphyria disorders in national health registries is enabling more structured management, ultimately driving market growth
- Collectively, rising awareness, enhanced diagnostic capabilities, and improved therapeutic pathways are contributing significantly to the increasing adoption of ALAD Porphyria Treatment solutions globally
Restraint/Challenge
Limited Treatment Availability and High Costs of Advanced Therapeutic Approaches
- A major challenge in the ALAD porphyria treatment market is the limited availability of targeted therapies, as the condition is exceptionally rare and requires specialized medical expertise for accurate diagnosis and management
- For instance, several countries in Asia, Africa, and Latin America still lack dedicated porphyria treatment centers, resulting in delayed detection, misdiagnosis, and restricted access to advanced therapies
- High costs associated with specialized diagnostic tools, enzyme assays, heme formulations, and emerging biologic or gene-based treatments also limit adoption, especially in low- and middle-income regions
- Furthermore, the rarity of ALAD Porphyria makes large-scale clinical trials difficult to conduct, slowing innovation and regulatory approvals for new therapies. Pharmaceutical companies often face challenges in investing in treatments for extremely small patient populations
- Limited physician awareness and inadequate training in rare metabolic disorders contribute to inconsistencies in clinical management, further restricting market expansion
- Achieving broader adoption will require increased research funding, development of cost-effective diagnostic methods, clinician training, and global expansion of rare-disease healthcare infrastructure
- Addressing these barriers is essential to improving accessibility, affordability, and patient outcomes in the ALAD Porphyria Treatment market
ALAD Porphyria Treatment Market Scope
The market is segmented on the basis of drug type, treatment type, dosage, route of administration, diagnosis, end-users, and distribution channel.
- By Drug Type
On the basis of drug type, the ALAD Porphyria Treatment market is segmented into anticonvulsant, NSAIDs, anti-emetic, enzyme inhibitors, and others. The anticonvulsant segment dominated the largest market revenue share of 38.4% in 2025, driven by its critical role in managing neurological manifestations associated with ALAD Porphyria, including seizure control and neuropathic symptoms. The segment benefits from widespread clinical familiarity and long-standing inclusion in treatment protocols. Growing availability of safer, newer-generation anticonvulsants supports physician adoption. Hospitals prefer anticonvulsants due to predictable dosing and favorable patient outcomes. Increased diagnostic rates of ALAD Porphyria also expand demand for symptomatic management drugs. International treatment guidelines highlight the importance of anticonvulsants in stabilizing acute episodes. Expanded reimbursement programs for chronic neurological conditions further strengthen market share. Rising global burden of porphyria-linked neuropathic pain increases reliance on these medications. Pharmaceutical companies continue to enhance formulations to reduce side effects. Increased accessibility across clinics and hospital pharmacies boosts usage. Anticonvulsants remain essential due to their immediate symptom-relief capability. Overall, this segment maintains strong clinical relevance, ensuring its dominance.
The NSAIDs segment is anticipated to witness the fastest CAGR of 19.7% from 2026 to 2033, driven by increasing utilization for managing abdominal pain, muscle aches, and inflammatory responses associated with ALAD Porphyria attacks. Their wide availability and strong physician preference for first-line symptomatic relief support rapid adoption. NSAIDs are cost-effective, making them accessible across developing and developed markets. The rising rate of outpatient management for mild to moderate episodes further drives NSAID demand. Improved formulations with reduced gastrointestinal risks increase patient compliance. Growing awareness campaigns about porphyria symptoms promote early symptomatic treatment, favoring NSAID uptake. Pharmacies globally report increased demand due to self-managed treatment preferences. Hospitals integrate NSAIDs into emergency room protocols for acute pain management. Manufacturers continue expanding production to meet rising demand. The segment also benefits from strong retail pharmacy penetration. As more patients seek non-opioid pain alternatives, NSAIDs become increasingly preferred. Overall, NSAIDs are expected to remain the fastest-growing treatment category.
- By Treatment Type
On the basis of treatment type, the ALAD Porphyria Treatment market is segmented into diet, genetic counseling, medication, and intravenous fluid replacement. The medication segment dominated the largest market revenue share of 46.1% in 2025, owing to its central role in preventing symptom escalation and stabilizing biochemical abnormalities associated with ALAD Porphyria. Physicians widely prescribe medications to manage neurological and gastrointestinal symptoms, driving consistent demand. Patient reliance on long-term drug therapy strengthens segment revenue. Pharmaceutical advancements introducing safer formulations enhance adoption. Global awareness programs about porphyria management have increased early diagnosis, leading to earlier medication initiation. Hospitals and clinics prioritize medication-based protocols due to proven clinical efficacy. The availability of multiple drug classes enables tailored therapy approaches. Insurance coverage for chronic drug therapy also boosts utilization. Growing prevalence of genetic disorders requiring drug therapy strengthens demand. Telemedicine expansion has increased prescription rates across remote regions. Medications remain the primary therapeutic intervention due to their role in preventing recurring attacks. Overall, the strong clinical dependence ensures segment leadership.
The intravenous fluid replacement segment is expected to witness the fastest CAGR of 20.2% from 2026 to 2033, driven by its vital function in stabilizing electrolyte imbalances and supporting metabolic correction during acute ALAD Porphyria episodes. Hospitals increasingly rely on IV fluids for rapid symptom management, particularly in severe attacks. Rising hospitalization rates for acute porphyria events fuel greater usage. Emergency departments include IV hydration as a first-line supportive therapy, boosting demand. Improved access to emergency care in developing regions contributes to segment expansion. IV fluids also support detoxification pathways that reduce symptom severity. Continuous advancements in hospital infrastructure improve availability. Increased clinician awareness of IV-based stabilization protocols raises utilization. The method offers fast, controlled administration, strengthening preference during acute episodes. Government programs expanding critical care access also support segment growth. As emergency management protocols become standardized globally, IV fluid use grows further. Overall, its essential role in acute care drives rapid CAGR.
- By Dosage
On the basis of dosage, the ALAD Porphyria Treatment market is segmented into tablet, solution, injection, and others. The tablet segment accounted for the largest market revenue share of 41.6% in 2025, driven by its ease of administration and strong patient preference for oral formulations in managing chronic symptoms associated with ALAD Porphyria. Tablets offer convenient dosing schedules, improving long-term adherence. Pharmaceutical companies continue expanding oral drug portfolios to accommodate rising chronic porphyria cases. Tablets also provide cost-effective therapy options, enhancing affordability across regions. Increased prescription rates in outpatient settings sustain demand. Clinicians favor tablets due to predictable absorption and minimal monitoring requirements. Retail pharmacies maintain high stock availability, improving accessibility. Patients undergoing long-term management often opt for oral medication due to routine integration. Insurance plans frequently reimburse oral medications more easily, supporting utilization. Advancements in oral formulations improve tolerability. Global expansion of telehealth-based prescriptions further increases tablet adoption. Overall, tablets remain the cornerstone for chronic symptom management.
The injection segment is expected to witness the fastest CAGR of 18.9% from 2026 to 2033, attributed to rising usage during acute episodes where rapid therapeutic action is required. Injections deliver faster symptom stabilization, making them preferred in emergency and hospital settings. Increased development of injectable formulations targeting metabolic correction enhances adoption. Clinicians rely on injections when oral therapy is insufficient for severe episodes. Growth in hospital admissions for acute porphyria attacks fuels demand. Improved storage and transport solutions expand availability in developing nations. Manufacturers invest in injectable innovations with improved safety profiles. IV and IM delivery methods provide precise dosing, preferred for severe symptom control. Emergency care units integrate injections into stabilization protocols. Government initiatives to modernize hospital facilities support wider use. Pharmaceutical pipelines show increased focus on injectable treatment development. Overall, injections continue gaining traction due to their critical role in acute management.
- By Route of Administration
On the basis of route of administration, the market is segmented into oral, intravenous, parenteral, and others. The oral segment held the largest market revenue share of 48.7% in 2025, supported by patient preference for non-invasive treatment methods and the extensive availability of oral formulations for chronic symptom management. Oral therapies ensure high patient convenience, improving adherence. Clinicians frequently prescribe oral drugs due to ease of long-term use. Pharmaceutical companies prioritize oral formulations due to strong market demand. The segment also benefits from large-scale retail pharmacy distribution. Teleconsultations have increased oral prescription frequency. Oral therapies are widely reimbursed, expanding their use. Patients managing mild-to-moderate symptoms rely heavily on oral regimens. Improved oral drug formulations with enhanced absorption support segment growth. Hospitals and clinics utilize oral medications in transition care following acute episodes. Global awareness of porphyria management promotes early initiation of oral therapy. Overall, oral administration remains dominant in chronic care.
The intravenous segment is expected to witness the fastest CAGR of 20.8% from 2026 to 2033, driven by its essential role in acute ALAD Porphyria management requiring rapid biochemical stabilization. IV administration ensures immediate therapeutic effect, vital during severe attacks. Hospitals rely heavily on IV treatment protocols for emergency care. Advances in infusion technology improve safety and efficiency. Increased hospitalization rates support demand for IV administration. Specialized metabolic therapies delivered through IV routes strengthen adoption. Growing critical care infrastructure in emerging economies boosts utilization. Training programs for emergency clinicians enhance use of IV methods. Improved availability of infusion centers supports wider access. Pharmaceutical development of IV-compatible formulations expands treatment choices. Patients with severe episodes often require IV therapy for effective stabilization. Overall, IV administration’s life-saving importance drives its rapid growth.
- By Diagnosis
On the basis of diagnosis, the ALAD Porphyria Treatment market is segmented into molecular genetic testing, specialised tests, and others. Molecular genetic testing dominated the largest market revenue share of 52.3% in 2025, driven by its accuracy in detecting genetic mutations responsible for ALAD Porphyria. Clinicians increasingly rely on genetic confirmation for precise diagnosis. Advancements in sequencing technologies improve sensitivity. The segment benefits from growing adoption of personalized medicine. Genetic testing enables early diagnosis, improving treatment outcomes. Many hospitals integrate genetic testing into standard porphyria diagnostic protocols. Governments expand funding for rare disease diagnosis, boosting testing use. Rising awareness among clinicians increases test prescriptions. Genetic counseling relies on testing outcomes, further supporting demand. Laboratories worldwide have improved capacity for molecular testing. Reduced test costs enhance affordability. Overall, genetic testing's diagnostic precision ensures its dominance.
Specialised tests are expected to witness the fastest CAGR of 17.5% from 2026 to 2033, fueled by increasing utilization of biochemical assays, enzyme activity tests, and porphyrin profile assessments. These tests provide detailed insights into disease severity and metabolic dysfunction. Hospitals rely on them for monitoring treatment response. Improvements in laboratory infrastructure support wider adoption. Specialized tests help distinguish between various porphyria subtypes, improving clinical accuracy. Increased clinician awareness promotes frequent utilization. Research institutions use these tests for monitoring genetic variants. Enhanced diagnostic sensitivity drives preference for metabolic profiling. Government rare-disease programs emphasize biochemical diagnostics. Improved reagent availability expands access in developing regions. Patients undergoing therapy require periodic assessments, supporting recurring demand. Overall, specialized tests grow rapidly due to their expanding clinical relevance.
- By End-Users
On the basis of end-users, the ALAD Porphyria Treatment market is segmented into clinic, hospital, and others. The hospital segment accounted for the largest market revenue share of 55.4% in 2025, attributed to the high rate of hospitalization during acute ALAD Porphyria attacks requiring intensive monitoring and rapid intervention. Hospitals provide advanced diagnostic tools and emergency care infrastructure. Availability of multidisciplinary teams enhances treatment effectiveness. Increased investment in specialized metabolic units supports segment growth. Hospitals manage the majority of severe cases, sustaining high demand. Government funding for rare disease treatment strengthens hospital capacity. Access to IV therapies and critical care reinforces dominance. Hospitals also serve as primary centers for genetic testing. Rising diagnostic awareness increases hospital visits. Specialized porphyria programs further boost segment leadership. Overall, hospitals remain central to acute care delivery.
The clinic segment is expected to witness the fastest CAGR of 18.4% from 2026 to 2033, supported by expanding outpatient management of chronic porphyria symptoms. Clinics provide accessible follow-up care and routine monitoring. Growth in primary healthcare centers boosts service availability. Clinics increasingly adopt genetic testing referrals, enhancing utilization. Rising awareness of early symptom identification increases patient visits. Physicians prefer clinic settings for long-term medication management. Telemedicine integration improves clinic reach. Enhanced diagnostic equipment availability strengthens adoption. Clinics offer cost-effective care options compared to hospitals. Patients seek regular monitoring for symptom progression, supporting demand. Growing population access to community health centers accelerates segment expansion. Overall, clinics grow rapidly due to expanding chronic care needs.
- By Distribution Channel
On the basis of distribution channel, the ALAD Porphyria Treatment market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. Hospital pharmacy dominated the largest market revenue share of 49.2% in 2025, driven by the high volume of medications and IV treatments dispensed during acute porphyria admissions. Hospitals maintain priority access to specialized therapies. Critical care protocols rely on rapid drug dispensing capabilities. Increased hospitalization rates support pharmacy demand. Hospitals also distribute medications for transition care following stabilization. Government programs supply rare disease medications through hospital channels. Pharmacists in hospitals closely monitor drug interactions, supporting compliance. Genetic testing outcomes often lead to immediate hospital-based treatment initiation. Hospitals maintain extensive inventories of emergency and injectable drugs. Improved hospital pharmacy infrastructure enhances operational efficiency. Patient trust in hospital-dispensed medications strengthens preference. Overall, hospital pharmacies remain essential for acute and specialized therapy delivery.
Online pharmacy is expected to witness the fastest CAGR of 21.3% from 2026 to 2033, propelled by rising digital penetration and increasing preference for home delivery of chronic porphyria medications. Online platforms offer convenience and broad product availability. Growing e-health adoption increases online prescription refills. Discounted pricing models attract cost-sensitive patients. Telemedicine growth boosts online prescription onboarding. Improved digital payment systems enhance accessibility. Chronic patients prefer online channels for long-term medication continuity. Platforms offer automated refill reminders, improving adherence. Expanding logistics networks reduce delivery times. Online pharmacies also improve access in rural areas. Regulatory reforms supporting e-pharmacy expansion boost adoption. Overall, digital transformation drives rapid online pharmacy growth.
ALAD Porphyria Treatment Market Regional Analysis
- North America dominated the ALAD Porphyria Treatment market with the largest revenue share of 40.00% in 2025
- Characterized by strong research infrastructure, high healthcare spending, and the presence of leading biopharmaceutical companies
- The region continues to advance in genetic testing, enzyme-based therapies, and clinical trial participation, which significantly boosts treatment accessibility and innovation
U.S. ALAD Porphyria Treatment Market Insight
The U.S. ALAD porphyria treatment market captured the largest revenue share of its region in 2025, driven by rapid adoption of advanced diagnostic tools, expanding patient registries, and increased focus on rare disease management. Strong investments in personalized medicine, widespread availability of specialized treatment centers, and ongoing development of new enzyme-targeted and genetic therapies further support market growth. Increased awareness campaigns and improved reimbursement pathways continue to enhance treatment adoption across hospitals and clinics.
Europe ALAD Porphyria Treatment Market Insight
The Europe ALAD porphyria treatment market is projected to expand at a substantial CAGR throughout the forecast period, fueled by strict healthcare regulations, rising focus on rare disease diagnosis, and growing adoption of molecular genetic testing. Increasing clinical research activities, improved access to porphyria specialists, and government-backed rare disease programs support market expansion across residential healthcare settings, outpatient facilities, and specialized diagnostic centers.
U.K. ALAD Porphyria Treatment Market Insight
The U.K. ALAD porphyria treatment market is expected to grow at a noteworthy CAGR due to increasing investments in precision medicine, strong genetic screening programs, and enhanced patient monitoring systems. Public health initiatives promoting rare disease awareness, combined with improved access to enzyme inhibitors and supportive therapies, continue to drive treatment uptake among both hospitals and specialized clinics.
Germany ALAD Porphyria Treatment Market Insight
The Germany ALAD porphyria treatment market is projected to expand at a considerable CAGR, supported by advanced healthcare infrastructure, strong biotechnology research, and rising demand for innovative diagnostic procedures. Germany’s focus on early identification of metabolic disorders and its accelerated adoption of enzyme-replacement and genetic testing platforms foster significant market growth.
Asia-Pacific ALAD Porphyria Treatment Market Insight
The Asia-Pacific ALAD porphyria treatment market is poised to grow at the fastest CAGR during the forecast period, driven by increasing healthcare modernization, rising investments in rare disease diagnosis, and expanding awareness programs led by medical organizations and government bodies. Countries such as China, Japan, and India are rapidly improving access to diagnostic technologies, specialist physicians, and advanced therapeutic options.
Japan ALAD Porphyria Treatment Market Insight
The Japan ALAD porphyria treatment market is experiencing steady momentum due to rising adoption of high-precision diagnostic tools, strong government support for rare disease research, and increasing demand for convenient treatment pathways. Japan’s focus on advanced molecular testing, alongside its aging population, is contributing to greater utilization of enzyme- and gene-based therapeutic solutions.
China ALAD Porphyria Treatment Market Insight
The China ALAD porphyria treatment market accounted for the largest market revenue share in Asia-Pacific in 2025, supported by rapid healthcare digitalization, expanding diagnostic capabilities, and strong domestic pharmaceutical manufacturing. Increasing awareness of porphyria disorders, growth in genomic testing adoption, and rising government initiatives for rare disease treatment are driving significant expansion of treatment availability across hospitals and specialty clinics.
ALAD Porphyria Treatment Market Share
The ALAD Porphyria Treatment industry is primarily led by well-established companies, including:
- Alnylam Pharmaceuticals (U.S.)
- Recordati Rare Diseases (Italy)
- Pfizer (U.S.)
- Roche Diagnostics (Switzerland)
- Thermo Fisher Scientific (U.S.)
- Quest Diagnostics (U.S.)
- LabCorp (U.S.)
- Agilent Technologies (U.S.)
- Bio-Rad Laboratories (U.S.)
- Siemens Healthineers (Germany)
- PerkinElmer (U.S.)
- Eurofins Scientific (Luxembourg)
- Beijing Hotgen Biotech (China)
- Abbott Laboratories (U.S.)
Latest Developments in Global ALAD Porphyria Treatment Market
- In August 2022, Frontiers in Genetics published a case report describing a patient with ALAD-deficiency porphyria who showed a lack of response to givosiran (Givlaari®), highlighting that RNAi therapy effective in common acute hepatic porphyrias may not reliably prevent attacks in ADP and underscoring the need for ADP-specific clinical data
- In October 2022, San Juan et al. published a Biochemistry study (Epub Oct 14, print Nov 1, 2022) showing that porphobilinogen inhibits ALAD activity at low concentrations, a mechanistic finding that helped explain ALA accumulation in acute porphyrias and guided researchers toward new biochemical and therapeutic hypotheses for ALAD-related disease
- In October 2024, an open-label 48-month follow-up study of givosiran (RNAi therapy for acute hepatic porphyrias) reported sustained reductions in attacks and continued safety signals, providing longer-term evidence for disease-modifying RNAi approaches in the broader acute porphyria field (while also motivating researchers to investigate how these findings apply — or do not apply — to the ultrarare ADP subtype)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.