Global Antihistamine Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
305.85 Billion
USD
620.71 Billion
2024
2032
USD
305.85 Billion
USD
620.71 Billion
2024
2032
| 2025 –2032 | |
| USD 305.85 Billion | |
| USD 620.71 Billion | |
|
|
|
|
Antihistamine Drugs Market Size
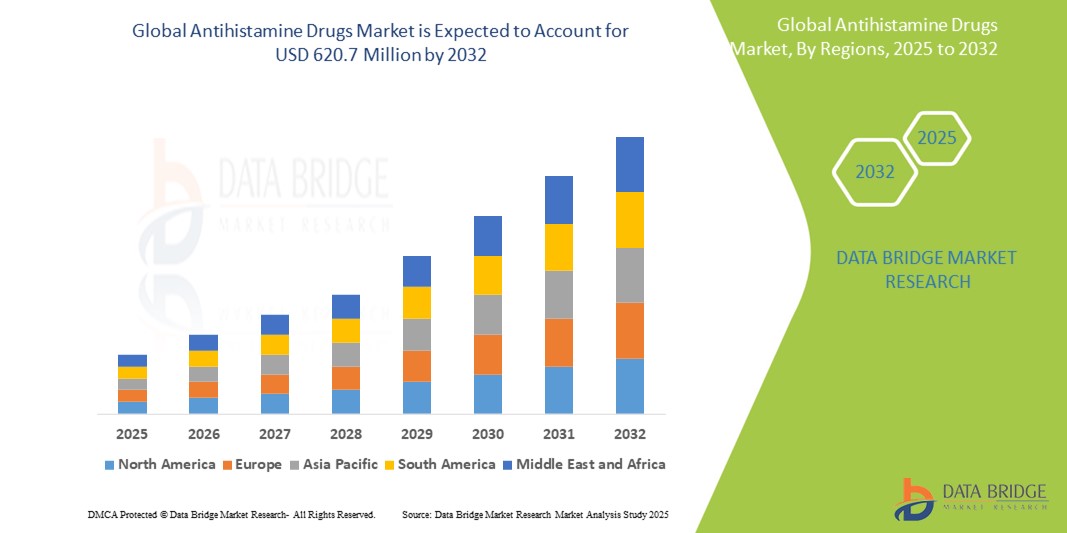
- The global antihistamine drugs market size was valued at USD 305.85 million in 2024 and is expected to reach USD 620.71 million by 2032, at a CAGR of 9.25% during the forecast period
- The market growth is largely driven by the increasing prevalence of allergic conditions such as allergic rhinitis, urticaria, and atopic dermatitis, coupled with heightened awareness and diagnosis rates across both developed and emerging regions
- Furthermore, growing demand for over-the-counter (OTC) antihistamines, advancements in second- and third-generation antihistamines with fewer sedative effects, and the expansion of e-pharmacy platforms are establishing antihistamines as a frontline therapeutic option. These converging factors are accelerating the adoption of antihistamine medications, thereby significantly boosting the industry's growth
Antihistamine Drugs Market Analysis
- Antihistamine drugs, used to block or reduce histamine-mediated allergic reactions, are becoming essential components in managing a wide range of allergic conditions such as hay fever, urticaria, and anaphylaxis in both acute and chronic care settings due to their rapid onset of action and broad therapeutic applicability
- The escalating demand for antihistamines is primarily fueled by the rising prevalence of allergic disorders globally, increasing self-medication trends, and growing awareness of allergy management solutions, especially non-sedating second-generation formulations
- North America dominated the antihistamine drugs market with the largest revenue share of 39.2% in 2024, characterized by high allergy prevalence, strong over-the-counter drug adoption, and the presence of major pharmaceutical companies, with the U.S. witnessing significant growth due to seasonal allergy peaks and consumer shift toward non-prescription antihistamines
- Asia-Pacific is expected to be the fastest growing region in the antihistamine drugs market during the forecast period due to increasing healthcare access, rising allergic disease incidence, and growing pharmaceutical investments
- The oral segment dominated the antihistamine drugs market with a market share of 61.9% in 2024, driven by its convenience, rapid symptom relief, and broad availability across prescription and non-prescription channels
Report Scope and Antihistamine Drugs Market Segmentation
|
Attributes |
Antihistamine Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Antihistamine Drugs Market Trends
“Shift Toward Second-Generation and Non-Sedating Formulations”
- A significant and accelerating trend in the global antihistamine drugs market is the growing preference for second-generation antihistamines due to their reduced sedative effects and improved safety profiles. These newer formulations are increasingly favored by both healthcare providers and patients for long-term allergy management
- For instance, drugs such as loratadine (Claritin), fexofenadine (Allegra), and cetirizine (Zyrtec) are widely used for their minimal central nervous system penetration, resulting in fewer drowsiness-related side effects, thus enhancing patient compliance, especially in daily or work-related environments
- Pharmaceutical advancements are driving sustained innovation in antihistamine delivery methods, including extended-release tablets and orally disintegrating formulations. Companies such as Johnson & Johnson and Sanofi continue to invest in research that enhances bioavailability and patient convenience
- The OTC availability of many non-sedating antihistamines further boosts their accessibility and fosters self-medication trends, particularly in regions with high allergy incidence and increasing consumer health awareness
- Moreover, combination therapies that integrate antihistamines with decongestants or corticosteroids are gaining traction for enhanced efficacy in treating multi-symptom allergies. These novel combinations are reshaping consumer expectations, pushing drugmakers to deliver more comprehensive allergy relief solutions
- The trend toward non-sedating, fast-acting, and patient-friendly antihistamines is fundamentally reshaping the allergy therapeutics landscape, as both healthcare systems and consumers demand safer, more convenient, and effective treatment options
Antihistamine Drugs Market Dynamics
Driver
“Rising Allergy Prevalence and Self-Medication Practices”
- The rising global prevalence of allergic conditions such as rhinitis, conjunctivitis, urticaria, and food allergies is a major factor fueling the demand for antihistamine drugs. Lifestyle changes, urban pollution, and genetic predisposition contribute to this rising incidence, prompting consistent demand for effective symptomatic relief
- For instance, the World Allergy Organization estimates that over 20–30% of the global population now suffers from allergic rhinitis, driving both prescription and OTC antihistamine drug sales worldwide
- Increasing patient awareness and the widespread availability of OTC antihistamines have led to a surge in self-medication, especially for mild-to-moderate allergies. Retail pharmacy expansion and e-commerce platforms have further facilitated access to these drugs
- In addition, advancements in antihistamine formulations that ensure faster onset of action and fewer side effects are improving patient adherence and satisfaction, encouraging continued market growth
Restraint/Challenge
“Side Effects and Regulatory Limitations on Certain Formulations”
- Despite their widespread use, antihistamines—particularly first-generation formulations—pose risks such as drowsiness, dizziness, and impaired motor function, which can limit their use in working adults and the elderly. These adverse effects remain a significant restraint to broader usage
- For instance, first-generation antihistamines such as diphenhydramine and chlorpheniramine are still used globally but are often accompanied by warnings about sedation and impaired alertness, which restrict their usage in certain demographics
- In addition, regulatory bodies in regions such as the EU and North America enforce stringent safety evaluations and restrictions on certain compounds due to potential adverse events or misuse
- Moreover, inappropriate use, such as overuse of OTC antihistamines without medical guidance, can lead to drug resistance or rebound symptoms. These concerns necessitate continuous consumer education and careful monitoring by regulatory authorities
- Overcoming these challenges requires stronger post-marketing surveillance, the development of safer formulations, and public health initiatives to guide responsible usage, ensuring sustainable growth of the antihistamine drugs market
Antihistamine Drugs Market Scope
The market is segmented on the basis of type, indication, dosage form, route of administration, end-users, and distribution channel.
- By Type
On the basis of type, the antihistamine drugs market is segmented into prescription-based and over-the-counter (OTC). The OTC segment dominated the market with the largest market revenue share in 2024, driven by the widespread availability and ease of access to antihistamines for common allergic symptoms such as hay fever and urticaria. Consumers prefer OTC options due to their convenience, cost-effectiveness, and the growing trend of self-medication. Key OTC drugs such as loratadine and cetirizine continue to witness strong sales through retail and online pharmacies.
The prescription-based segment is anticipated to witness steady growth from 2025 to 2032, fueled by rising cases of chronic allergic conditions and severe allergic reactions requiring stronger or combination therapies. Prescription formulations often target specific conditions and include higher dosages or dual-action mechanisms not available OTC.
- By Indication
On the basis of indication, the antihistamine drugs market is segmented into urticaria, allergy, dermatitis, and others. The allergy segment held the largest market share in 2024, attributed to the high global prevalence of seasonal allergic rhinitis, food allergies, and respiratory allergies. Increasing air pollution and climate-related changes contribute to the expanding patient pool.
The urticaria segment is expected to grow at the fastest pace during the forecast period, due to rising awareness and diagnosis rates, particularly for chronic spontaneous urticaria (CSU), which requires consistent therapeutic management through antihistamines.
- By Dosage Form
On the basis of dosage form, the antihistamine drugs market is segmented into tablets, capsules, and others. The tablet segment dominated the market with the highest revenue share in 2024, owing to its convenience, rapid onset of action, and widespread availability in both OTC and prescription forms.
The capsule segment is projected to witness fastest growth during the forecast period, supported by innovations in extended-release formulations that improve dosing compliance and reduce frequency of intake.
- By Route Of Administration
On the basis of route of administration, the antihistamine drugs market is segmented into oral, parenteral, and others. The oral segment held the dominant share of 61.9% in 2024 due to its non-invasive nature, ease of administration, and suitability for self-treatment. Oral antihistamines are commonly preferred for conditions such as allergic rhinitis and urticaria.
The parenteral segment is expected to register growth during forecast period in hospital and emergency settings where rapid action is needed for severe allergic reactions or anaphylaxis.
- By End User
On the basis of end-users, the market is segmented into hospitals, specialty clinics, homecare, and others. The hospitals segment led the market in 2024 due to the need for immediate and intensive care for acute allergic reactions and the availability of injectable antihistamines.
The homecare segment is expected to grow significantly over the forecast period, driven by increased consumer awareness and self-management of mild allergic conditions using OTC antihistamines and telehealth support
- By Distribution Channel
On the basis of distribution channel, the antihistamine drugs market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, and others. The retail pharmacy segment accounted for the largest market share in 2024, due to the ease of access to both OTC and prescription drugs, especially in urban and suburban areas.
The online pharmacy segment is expected to experience the fastest growth from 2025 to 2032, supported by the digital transformation of healthcare, rising smartphone penetration, and the convenience of home delivery services.
Antihistamine Drugs Market Regional Analysis
- North America dominated the antihistamine drugs market with the largest revenue share of 39.2% in 2024, characterized by high allergy prevalence, strong over-the-counter drug adoption, and the presence of major pharmaceutical companies, with the U.S. witnessing significant growth due to seasonal allergy peaks and consumer shift toward non-prescription antihistamines
- Consumers in the region prioritize fast-acting and accessible treatment options, leading to strong demand for both prescription and OTC formulations across retail and online pharmacies
- This dominant market position is further supported by high healthcare spending, increasing awareness of allergy management, and robust pharmaceutical distribution networks, making antihistamines a routine part of allergy care for both acute and chronic conditions
U.S. Antihistamine Drugs Market Insight
The U.S. antihistamine drugs market captured the largest revenue share of 79.2% in 2024 within North America, fueled by the high incidence of seasonal allergies, asthma, and other allergic conditions. Consumers increasingly seek fast, accessible relief through OTC and prescription antihistamines, supported by favorable reimbursement policies and a strong retail pharmacy network. The widespread availability of combination therapies and advanced formulations, along with proactive awareness campaigns on allergy management, further propels market growth.
Europe Antihistamine Drugs Market Insight
The Europe antihistamine drugs market is projected to grow at a steady CAGR throughout the forecast period, driven by rising environmental allergens and growing awareness of allergic conditions. Stringent healthcare regulations and high standards of medical care are fostering the uptake of prescription-based treatments. In addition, consumer preference for non-sedative, second-generation antihistamines is expanding the market, especially in countries such as Germany, France, and the U.K., where allergy diagnoses and treatment adherence are comparatively higher.
U.K. Antihistamine Drugs Market Insight
The U.K. antihistamine drugs market is anticipated to grow at a notable CAGR during the forecast period, attributed to a high prevalence of hay fever and skin allergies. Increased public health initiatives and consumer education on self-care contribute to growing OTC sales. The expansion of e-pharmacies, alongside robust NHS support for allergy treatment, enhances accessibility, especially during peak allergy seasons.
Germany Antihistamine Drugs Market Insight
The Germany antihistamine drugs market is expected to grow at a considerable CAGR during the forecast period, driven by the country’s high environmental allergen levels and strong regulatory oversight. German consumers favor non-drowsy and long-acting formulations, and the market benefits from physician-led allergy management and a well-established insurance system. Innovations in dual-action therapies and combination products are gaining traction in both hospital and retail channels.
Asia-Pacific Antihistamine Drugs Market Insight
The Asia-Pacific antihistamine drugs market is poised to grow at the fastest CAGR of 23.1% during the forecast period of 2025 to 2032, led by increasing pollution, urbanization, and rising awareness of allergic diseases in countries such as China, India, and Japan. Expanding healthcare infrastructure and growing access to OTC medications are propelling demand. Governments promoting universal healthcare access and the rise of e-pharmacies are further enhancing regional market penetration.
Japan Antihistamine Drugs Market Insight
The Japan antihistamine drugs market is experiencing significant growth, driven by a culture focused on early diagnosis, preventive care, and consumer demand for advanced therapies. High prevalence of cedar pollen-induced hay fever supports year-round demand. Japanese consumers favor second-generation antihistamines with minimal side effects, and digital health platforms are increasingly used to manage allergy symptoms and refill prescriptions conveniently.
India Antihistamine Drugs Market Insight
The India antihistamine drugs market accounted for the largest market revenue share in Asia-Pacific in 2024, propelled by increasing cases of respiratory and skin allergies, rapid urbanization, and an expanding pharmaceutical retail sector. High population density and rising pollution levels contribute to demand growth. The growing availability of affordable OTC antihistamines, supported by local manufacturers and government-backed healthcare initiatives, are key drivers expanding access to treatment across urban and rural regions.
Antihistamine Drugs Market Share
The antihistamine drugs industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sanofi (France)
- Akorn Operation Company LLC (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- AstraZeneca (U.K.)
- Johnson & Johnson Services, Inc. (U.S.)
- Bayer AG (Germany)
- Merck & Co., Inc. (U.S.)
- Prestige Consumer Healthcare Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Bristol-Myers Squibb Company (U.S.)
- Almirall, S.A (Spain)
- Zenomed Healthcare Private Limited (India)
- Cadila Pharmaceuticals (India)
What are the Recent Developments in Global Antihistamine Drugs Market?
- In April 2025, Morepen Laboratories, a leading active pharmaceutical ingredient (API) manufacturer, received approval from the Center for Drug Evaluation under China’s National Medical Products Administration (NMPA) for its anti-allergy API, Loratadine. This regulatory milestone is expected to enable Morepen to gain a substantial foothold in the Chinese market and further strengthen its position as a major global supplier of Loratadine
- In April 2025, the U.S. Food and Drug Administration (FDA) approved Dupixent (dupilumab) for the treatment of chronic spontaneous urticaria (CSU) in adults and adolescents aged 12 and older who continue to experience symptoms despite treatment with H1 antihistamines.
- In October 2024, Alembic Pharmaceuticals announced that it had received final approval from the U.S. Food and Drug Administration (US FDA) for its Abbreviated New Drug Application (ANDA) for Alcaftadine Ophthalmic Solution. The approved product is deemed therapeutically equivalent to the reference listed drug (RLD), Lastacaft Solution, developed by AbbVie Inc
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ANTIHISTAMINE DRUGS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ANTIHISTAMINE DRUGS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ANTIHISTAMINE DRUGS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 EPIDEMIOLOGY
11.1 INCIDENCE OF ALL BY GENDER
11.2 TREATMENT RATE
11.3 MORTALITY RATE
11.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
11.5 PATIENT TREATMENT SUCCESS RATES
12 REGULATORY COMPLIANCE
12.1 REGULATORY AUTHORITIES
12.2 REGULATORY CLASSIFICATIONS
12.2.1 CLASS I
12.2.2 CLASS II
12.2.3 CLASS III
12.3 REGULATORY SUBMISSIONS
12.4 INTERNATIONAL HARMONIZATION
12.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
12.6 REGULATORY CHALLENGES AND STRATEGIES
13 PIPELINE ANALYSIS
13.1 CLINICAL TRIALS AND PHASE ANALYSIS
13.2 DRUG THERAPY PIPELINE
13.3 PHASE III CANDIDATES
13.4 PHASE II CANDIDATES
13.5 PHASE I CANDIDATES
13.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR ANTIHISTAMINE DRUGS MARKET
Company Name Product Name
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE FOR ANTIHISTAMINE DRUGS MARKET
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved but Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE FOR ANTIHISTAMINE DRUGS MARKET
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE FOR ANTIHISTAMINE DRUGS MARKET
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR ANTIHISTAMINE DRUGS MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
14 REIMBURSEMENT FRAMEWORK
15 OPPUTUNITY MAP ANALYSIS
16 VALUE CHAIN ANALYSIS
17 HEALTHCARE ECONOMY
17.1 HEALTHCARE EXPENDITURE
17.2 CAPITAL EXPENDITURE
17.3 CAPEX TRENDS
17.4 CAPEX ALLOCATION
17.5 FUNDING SOURCES
17.6 INDUSTRY BENCHMARKS
17.7 GDP RATION IN OVERALL GDP
17.8 HEALTHCARE SYSTEM STRUCTURE
17.9 GOVERNMENT POLICIES
17.1 ECONOMIC DEVELOPMENT
18 GLOBAL ANTIHISTAMINE DRUGS MARKET, BY THERAPY TYPE
(NOTE: MARKET VALUE, MARKET VOLUME AND ASP WILL BE PROVIDED FOR ALL SEGMENTS AND SUB-SEGMENTS)
18.1 OVERVIEW
18.2 MONOTHERAPY
18.3 COMBINATION THERAPY
19 GLOBAL ANTIHISTAMINE DRUGS MARKET, BY DRUG CLASS
(NOTE: MARKET VALUE, MARKET VOLUME AND ASP WILL BE PROVIDED FOR ALL SEGMENTS AND SUB-SEGMENTS)
19.1 OVERVIEW
19.2 FIRST GENERATION ANTIHISTAMINE (SEDATING)
19.2.1 BROMPHENIRAMINE
19.2.1.1. BY STREGHT
19.2.1.1.1. 1MG/1ML
19.2.1.1.2. 2 MG/5ML
19.2.1.1.3. OTHERS
19.2.1.2. BY DRUG TYPE
19.2.1.2.1. BRANDED
19.2.1.2.2. GENERICS
19.2.1.3. OTHERS
19.2.2 CARBINOXAMINE MALEATE
19.2.2.1. BY STREGHT
19.2.2.1.1. 4 MG
19.2.2.1.2. 10 MG
19.2.2.1.3. OTHERS
19.2.2.2. BY DRUG TYPE
19.2.2.2.1. BRANDED
19.2.2.2.2. GENERICS
19.2.2.3. OTHERS
19.2.3 CHLORPHENIRAMINE
19.2.3.1. BY STREGHT
19.2.3.1.1. 4 MG
19.2.3.1.2. 8 MG
19.2.3.1.3. 10 MG
19.2.3.1.4. OTHERS
19.2.3.2. BY DRUG TYPE
19.2.3.2.1. BRANDED
19.2.3.2.2. GENERICS
19.2.3.3. OTHERS
19.2.4 DIPHENHYDRAMINE
19.2.4.1. BY STREGHT
19.2.4.1.1. 25 MG
19.2.4.1.2. 50 MG
19.2.4.1.3. OTHERS
19.2.4.2. BY DRUG TYPE
19.2.4.2.1. BRANDED
19.2.4.2.2. GENERICS
19.2.4.3. OTHERS
19.2.5 HYDROXYZINE
19.2.5.1. BY STREGHT
19.2.5.1.1. 10 MG
19.2.5.1.2. 20 MG
19.2.5.1.3. 50 MG
19.2.5.1.4. OTHERS
19.2.5.2. BY DRUG TYPE
19.2.5.2.1. BRANDED
19.2.5.2.2. GENERICS
19.2.5.3. OTHERS
19.2.6 TRIPROLIDINE
19.2.6.1. BY STREGHT
19.2.6.1.1. 0.5MG/ML
19.2.6.1.2. 0.625MG/ML
19.2.6.1.3. OTHERS
19.2.6.2. BY DRUG TYPE
19.2.6.2.1. BRANDED
19.2.6.2.2. GENERICS
19.2.6.3. OTHERS
19.2.7 CLEMASTINE
19.2.7.1. BY STREGHT
19.2.7.1.1. 1.34MG
19.2.7.1.2. 2.68MG
19.2.7.1.3. OTHERS
19.2.7.2. BY DRUG TYPE
19.2.7.2.1. BRANDED
19.2.7.2.2. GENERICS
19.2.7.3. OTHERS
19.2.8 KETOTIFEN
19.2.8.1. BY STREGHT
19.2.8.1.1. 1MG
19.2.8.1.2. OTHERS
19.2.8.2. BY DRUG TYPE
19.2.8.2.1. BRANDED
19.2.8.2.2. GENERICS
19.2.8.3. OTHERS
19.2.9 PROMETHAZINE HYDROCHLORIDE
19.2.9.1. BY STREGHT
19.2.9.1.1. 12.5MG
19.2.9.1.2. 25MG
19.2.9.1.3. 50MG
19.2.9.1.4. OTHERS
19.2.9.2. BY DRUG TYPE
19.2.9.2.1. BRANDED
19.2.9.2.2. GENERICS
19.2.9.3. OTHERS
19.2.10 PROMETHAZINE TEOCLATE
19.2.10.1. BY STREGHT
19.2.10.1.1. 1.34MG
19.2.10.1.2. 2.68MG
19.2.10.1.3. OTHERS
19.2.10.2. BY DRUG TYPE
19.2.10.2.1. BRANDED
19.2.10.2.2. GENERICS
19.2.10.3. OTHERS
19.2.11 CYPROHEPTADINE
19.2.11.1. BY STREGHT
19.2.11.1.1. 4 MG
19.2.11.1.2. OTHERS
19.2.11.2. BY DRUG TYPE
19.2.11.2.1. BRANDED
19.2.11.2.2. GENERICS
19.2.11.3. OTHERS
19.2.12 CINNARIZINE
19.2.12.1. BY STREGHT
19.2.12.1.1. 15 MG
19.2.12.1.2. 25 MG
19.2.12.1.3. OTHERS
19.2.12.2. BY DRUG TYPE
19.2.12.2.1. BRANDED
19.2.12.2.2. GENERICS
19.2.12.3. OTHERS
19.2.13 ALIMEMAZINE
19.2.13.1. BY STREGHT
19.2.13.1.1. 1 MG
19.2.13.1.2. 10 MG
19.2.13.1.3. OTHERS
19.2.13.2. BY DRUG TYPE
19.2.13.2.1. BRANDED
19.2.13.2.2. GENERICS
19.2.13.3. OTHERS
19.2.14 OTHERS
19.3 SECOND GENERATION ANTIHISTAMINES (NON SEDATING)
19.3.1 AZELASTINE
19.3.1.1. BY STREGHT
19.3.1.1.1. 0.1% SOLUTION
19.3.1.1.2. 0.15% SOLUTION
19.3.1.2. BY DRUG TYPE
19.3.1.2.1. BRANDED
19.3.1.2.2. GENERICS
19.3.1.3. OTHERS
19.3.2 OLOPATADINE
19.3.2.1. BY STREGHT
19.3.2.1.1. 0.1% SOLUTION
19.3.2.1.2. 0.2% SOLUTION
19.3.2.2. BY DRUG TYPE
19.3.2.2.1. BRANDED
19.3.2.2.2. GENERICS
19.3.2.3. OTHERS
19.3.3 ACRIVASTINE
19.3.3.1. BY STREGHT
19.3.3.1.1. 8 MG
19.3.3.1.2. 60 MG
19.3.3.2. BY DRUG TYPE
19.3.3.2.1. BRANDED
19.3.3.2.2. GENERICS
19.3.3.3. OTHERS
19.3.4 BILASTINE
19.3.4.1. BY STREGHT
19.3.4.1.1. 10 MG
19.3.4.1.2. 20 MG
19.3.4.1.3. OTHERS
19.3.4.2. BY DRUG TYPE
19.3.4.2.1. BRANDED
19.3.4.2.2. GENERICS
19.3.4.3. OTHERS
19.3.5 DESLORATADINE
19.3.5.1. BY STREGHT
19.3.5.1.1. 2.5 MG
19.3.5.1.2. 5 MG
19.3.5.1.3. OTHERS
19.3.5.2. BY DRUG TYPE
19.3.5.2.1. BRANDED
19.3.5.2.2. GENERICS
19.3.5.3. OTHERS
19.3.6 FEXOFENADINE
19.3.6.1. BY STREGHT
19.3.6.1.1. 30 MG
19.3.6.1.2. 60 MG
19.3.6.1.3. 180 MG
19.3.6.1.4. OTHERS
19.3.6.2. BY DRUG TYPE
19.3.6.2.1. BRANDED
19.3.6.2.2. GENERICS
19.3.6.3. OTHERS
19.3.7 CETIRIZINE
19.3.7.1. BY STREGHT
19.3.7.1.1. 5 MG
19.3.7.1.2. 10 MG
19.3.7.1.3. OTHERS
19.3.7.2. BY DRUG TYPE
19.3.7.2.1. BRANDED
19.3.7.2.2. GENERICS
19.3.7.3. OTHERS
19.3.8 LEVOCETIRIZINE
19.3.8.1. BY STREGHT
19.3.8.1.1. 5 MG
19.3.8.1.2. 2.5 MG
19.3.8.1.3. OTHERS
19.3.8.2. BY DRUG TYPE
19.3.8.2.1. BRANDED
19.3.8.2.2. GENERICS
19.3.8.3. OTHERS
19.3.9 LORATADINE
19.3.9.1. BY STREGHT
19.3.9.1.1. 5 MG
19.3.9.1.2. 10 MG
19.3.9.1.3. OTHERS
19.3.9.2. BY DRUG TYPE
19.3.9.2.1. BRANDED
19.3.9.2.2. GENERICS
19.3.9.3. OTHERS
19.3.10 MIZOLASTINE
19.3.10.1. BY STREGHT
19.3.10.1.1. 10 MG
19.3.10.1.2. OTHERS
19.3.10.2. BY DRUG TYPE
19.3.10.2.1. BRANDED
19.3.10.2.2. GENERICS
19.3.10.3. OTHERS
19.3.11 RUPATADINE
19.3.11.1. BY STREGHT
19.3.11.1.1. 10 MG
19.3.11.1.2. OTHERS
19.3.11.2. BY DRUG TYPE
19.3.11.2.1. BRANDED
19.3.11.2.2. GENERICS
19.3.11.3. OTHERS
19.3.12 OTHERS
19.4 OTHER
20 GLOBAL ANTIHISTAMINE DRUGS MARKET, BY ROUTE OF ADMINISTRATION
20.1 OVERVIEW
20.2 ORAL
20.2.1 CAPSULES
20.2.2 TABLETS
20.2.3 OTHERS
20.3 PARENTERAL
20.3.1 INTRAVENEOUS
20.3.2 SUBCUTANEOUS
20.3.3 OTHERS
20.4 INTRANASAL
20.5 INTRAOCULAR
20.6 OTHERS
21 GLOBAL ANTIHISTAMINE DRUGS MARKET, BY DRUG TYPE
21.1 OVERVIEW
21.2 BRANDED
21.2.1 ZYRTEC D
21.2.2 XYZAL
21.2.3 CLARINEX
21.2.4 OTHERS
21.3 GENERICS
22 GLOBAL ANTIHISTAMINE DRUGS MARKET, BY PRESCRIPTION MODE
22.1 OVERVIEW
22.2 PRESCRIPTION-BASED
22.2.1 ASTELIN
22.2.2 ARBINOXA
22.2.3 OTHERS
22.3 OVER-THE-COUNTER (OTC)
23 GLOBAL ANTIHISTAMINE DRUGS MARKET, BY POPULATION TYPE
23.1 OVERVIEW
23.2 BELOW 6 YEARS
23.3 6-15 YEARS
23.4 ABOVE 15 YEARS
24 GLOBAL ANTIHISTAMINE DRUGS MARKET, BY GENDER
24.1 OVERVIEW
24.2 MALE
24.2.1 BELOW 6 YEARS
24.2.2 6-15 YEARS
24.2.3 ABOVE 15 YEARS
24.3 FEMALE
24.3.1 BELOW 6 YEARS
24.3.2 6-15 YEARS
24.3.3 ABOVE 15 YEARS
25 GLOBAL ANTIHISTAMINE DRUGS MARKET, BY INDICATION
25.1 OVERVIEW
25.2 URTICARIA
25.3 ALLERGY
25.3.1 ALLERGIC RHINITIS
25.3.2 ALLERGIC CONJUNCTIVITIS
25.3.3 ALLERGIC DERMATOLOGICAL REACTION
25.3.4 OTHERS
25.4 DERMATITIS
25.5 SINUSITIS
25.6 PEPTIC ULCER
25.7 ZOLLINGER ELLISON SYNDROME
25.8 OTHER
26 GLOBAL ANTIHISTAMINE DRUGS MARKET, BY END USER
26.1 OVERVIEW
26.2 HOSPITALS
26.2.1 PUBLIC
26.2.2 PRIVATE
26.3 SPECIALTY CLINICS
26.4 HOME HEALTHCARE
26.5 ACADEMIC AND RESEARCH INSTITUTE
26.6 OTHERS
27 GLOBAL ANTIHISTAMINE DRUGS MARKET, BY DISTRIBUTION CHANNEL
27.1 OVERVIEW
27.2 DIRECT TENDER
27.3 RETAIL SALES
27.3.1 ONLINE PHARMACY
27.3.2 OFFLINE PHARMACY
27.4 OTHERS
28 GLOBAL ANTIHISTAMINE DRUGS MARKET, COMPANY LANDSCAPE
28.1 COMPANY SHARE ANALYSIS: GLOBAL
28.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
28.3 COMPANY SHARE ANALYSIS: EUROPE
28.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
28.5 MERGERS & ACQUISITIONS
28.6 NEW PRODUCT DEVELOPMENT & APPROVALS
28.7 EXPANSIONS
28.8 REGULATORY CHANGES
28.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
29 GLOBAL ANTIHISTAMINE DRUGS MARKET, BY REGION
GLOBAL ANTIHISTAMINE DRUGS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
29.1 NORTH AMERICA
29.1.1 U.S.
29.1.2 CANADA
29.1.3 MEXICO
29.2 EUROPE
29.2.1 GERMANY
29.2.2 U.K.
29.2.3 ITALY
29.2.4 FRANCE
29.2.5 SPAIN
29.2.6 RUSSIA
29.2.7 SWITZERLAND
29.2.8 TURKEY
29.2.9 BELGIUM
29.2.10 NETHERLANDS
29.2.11 DENMARK
29.2.12 SWEDEN
29.2.13 POLAND
29.2.14 NORWAY
29.2.15 FINLAND
29.2.16 REST OF EUROPE
29.3 ASIA-PACIFIC
29.3.1 JAPAN
29.3.2 CHINA
29.3.3 SOUTH KOREA
29.3.4 INDIA
29.3.5 SINGAPORE
29.3.6 THAILAND
29.3.7 INDONESIA
29.3.8 MALAYSIA
29.3.9 PHILIPPINES
29.3.10 AUSTRALIA
29.3.11 NEW ZEALAND
29.3.12 VIETNAM
29.3.13 TAIWAN
29.3.14 REST OF ASIA-PACIFIC
29.4 SOUTH AMERICA
29.4.1 BRAZIL
29.4.2 ARGENTINA
29.4.3 REST OF SOUTH AMERICA
29.5 MIDDLE EAST AND AFRICA
29.5.1 SOUTH AFRICA
29.5.2 EGYPT
29.5.3 BAHRAIN
29.5.4 UNITED ARAB EMIRATES
29.5.5 KUWAIT
29.5.6 OMAN
29.5.7 QATAR
29.5.8 SAUDI ARABIA
29.5.9 REST OF MEA
29.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
30 GLOBAL ANTIHISTAMINE DRUGS MARKET, SWOT AND DBMR ANALYSIS
31 GLOBAL ANTIHISTAMINE DRUGS MARKET, COMPANY PROFILE
31.1 VITARIS INC.
31.1.1 COMPANY OVERVIEW
31.1.2 REVENUE ANALYSIS
31.1.3 GEOGRAPHIC PRESENCE
31.1.4 PRODUCT PORTFOLIO
31.1.5 RECENT DEVELOPMENTS
31.2 JOHNSON & JOHNSON SERVICES, INC.
31.2.1 COMPANY OVERVIEW
31.2.2 REVENUE ANALYSIS
31.2.3 GEOGRAPHIC PRESENCE
31.2.4 PRODUCT PORTFOLIO
31.2.5 RECENT DEVELOPMENTS
31.3 ACTAVIS ELIZABETH LLC
31.3.1 COMPANY OVERVIEW
31.3.2 REVENUE ANALYSIS
31.3.3 GEOGRAPHIC PRESENCE
31.3.4 PRODUCT PORTFOLIO
31.3.5 RECENT DEVELOPMENTS
31.4 APOTEX INC.
31.4.1 COMPANY OVERVIEW
31.4.2 REVENUE ANALYSIS
31.4.3 GEOGRAPHIC PRESENCE
31.4.4 PRODUCT PORTFOLIO
31.4.5 RECENT DEVELOPMENTS
31.5 CARACO PHARMACEUTICAL LABORATORIES, LTD (SUN PHARMACEUTICAL INDUSTRIES LTD)
31.5.1 COMPANY OVERVIEW
31.5.2 REVENUE ANALYSIS
31.5.3 GEOGRAPHIC PRESENCE
31.5.4 PRODUCT PORTFOLIO
31.5.5 RECENT DEVELOPMENTS
31.6 DR. REDDY’S LABORATORIES LTD.
31.6.1 COMPANY OVERVIEW
31.6.2 REVENUE ANALYSIS
31.6.3 GEOGRAPHIC PRESENCE
31.6.4 PRODUCT PORTFOLIO
31.6.5 RECENT DEVELOPMENTS
31.7 PERRIGO COMPANY PLC
31.7.1 COMPANY OVERVIEW
31.7.2 REVENUE ANALYSIS
31.7.3 GEOGRAPHIC PRESENCE
31.7.4 PRODUCT PORTFOLIO
31.7.5 RECENT DEVELOPMENTS
31.8 SANDOZ AG (NOVARTIS AG)
31.8.1 COMPANY OVERVIEW
31.8.2 REVENUE ANALYSIS
31.8.3 GEOGRAPHIC PRESENCE
31.8.4 PRODUCT PORTFOLIO
31.8.5 RECENT DEVELOPMENTS
31.9 WOCKHARDT USA
31.9.1 COMPANY OVERVIEW
31.9.2 REVENUE ANALYSIS
31.9.3 GEOGRAPHIC PRESENCE
31.9.4 PRODUCT PORTFOLIO
31.9.5 RECENT DEVELOPMENTS
31.1 PFIZER INC.
31.10.1 COMPANY OVERVIEW
31.10.2 REVENUE ANALYSIS
31.10.3 GEOGRAPHIC PRESENCE
31.10.4 PRODUCT PORTFOLIO
31.10.5 RECENT DEVELOPMENTS
31.11 TRINITY PHARMA (PTY) LTD.
31.11.1 COMPANY OVERVIEW
31.11.2 REVENUE ANALYSIS
31.11.3 GEOGRAPHIC PRESENCE
31.11.4 PRODUCT PORTFOLIO
31.11.5 RECENT DEVELOPMENTS
31.12 AVET PHARMACEUTICALS INC
31.12.1 COMPANY OVERVIEW
31.12.2 REVENUE ANALYSIS
31.12.3 GEOGRAPHIC PRESENCE
31.12.4 PRODUCT PORTFOLIO
31.12.5 RECENT DEVELOPMENTS
31.13 TEVA PHARMACEUTICALS USA, INC.
31.13.1 COMPANY OVERVIEW
31.13.2 REVENUE ANALYSIS
31.13.3 GEOGRAPHIC PRESENCE
31.13.4 PRODUCT PORTFOLIO
31.13.5 RECENT DEVELOPMENTS
31.14 CIPLA
31.14.1 COMPANY OVERVIEW
31.14.2 REVENUE ANALYSIS
31.14.3 GEOGRAPHIC PRESENCE
31.14.4 PRODUCT PORTFOLIO
31.14.5 RECENT DEVELOPMENTS
31.15 TAJ PHARMA GROUP
31.15.1 COMPANY OVERVIEW
31.15.2 REVENUE ANALYSIS
31.15.3 GEOGRAPHIC PRESENCE
31.15.4 PRODUCT PORTFOLIO
31.15.5 RECENT DEVELOPMENTS
31.16 UCB S.A.
31.16.1 COMPANY OVERVIEW
31.16.2 REVENUE ANALYSIS
31.16.3 GEOGRAPHIC PRESENCE
31.16.4 PRODUCT PORTFOLIO
31.17 CAMBER PHARMACEUTICALS, INC
31.17.1 COMPANY OVERVIEW
31.17.2 REVENUE ANALYSIS
31.17.3 GEOGRAPHIC PRESENCE
31.17.4 PRODUCT PORTFOLIO
31.18 GLENMARK PHARMACEUTICALS INC., USA
31.18.1 COMPANY OVERVIEW
31.18.2 REVENUE ANALYSIS
31.18.3 GEOGRAPHIC PRESENCE
31.18.4 PRODUCT PORTFOLIO
31.19 WELLONA PHARMA
31.19.1 COMPANY OVERVIEW
31.19.2 REVENUE ANALYSIS
31.19.3 GEOGRAPHIC PRESENCE
31.19.4 PRODUCT PORTFOLIO
31.2 ADVACARE PHARMA
31.20.1 COMPANY OVERVIEW
31.20.2 REVENUE ANALYSIS
31.20.3 GEOGRAPHIC PRESENCE
31.20.4 PRODUCT PORTFOLIO
31.21 ERIS LIFESCIENCES
31.21.1 COMPANY OVERVIEW
31.21.2 REVENUE ANALYSIS
31.21.3 GEOGRAPHIC PRESENCE
31.21.4 PRODUCT PORTFOLIO
31.21.5 RECENT DEVELOPMENTS
31.22 SANOFI
31.22.1 COMPANY OVERVIEW
31.22.2 REVENUE ANALYSIS
31.22.3 GEOGRAPHIC PRESENCE
31.22.4 PRODUCT PORTFOLIO
31.22.5 RECENT DEVELOPMENTS
31.23 ADEN HEALTHCARE
31.23.1 COMPANY OVERVIEW
31.23.2 REVENUE ANALYSIS
31.23.3 GEOGRAPHIC PRESENCE
31.23.4 PRODUCT PORTFOLIO
31.23.5 RECENT DEVELOPMENTS
31.24 ORGANON GROUP OF COMPANIES
31.24.1 COMPANY OVERVIEW
31.24.2 REVENUE ANALYSIS
31.24.3 GEOGRAPHIC PRESENCE
31.24.4 PRODUCT PORTFOLIO
31.24.5 RECENT DEVELOPMENTS
31.25 MERCK & CO., INC
31.25.1 COMPANY OVERVIEW
31.25.2 REVENUE ANALYSIS
31.25.3 GEOGRAPHIC PRESENCE
31.25.4 PRODUCT PORTFOLIO
31.25.5 RECENT DEVELOPMENTS
31.26 LUPIN PHARMACEUTICALS, INC.
31.26.1 COMPANY OVERVIEW
31.26.2 REVENUE ANALYSIS
31.26.3 GEOGRAPHIC PRESENCE
31.26.4 PRODUCT PORTFOLIO
31.26.5 RECENT DEVELOPMENTS
31.27 ORBION PHARMACEUTICALS PRIVATE LTD
31.27.1 COMPANY OVERVIEW
31.27.2 REVENUE ANALYSIS
31.27.3 GEOGRAPHIC PRESENCE
31.27.4 PRODUCT PORTFOLIO
31.27.5 RECENT DEVELOPMENTS
31.28 SCIEGEN PHARMACEUTICALS INC
31.28.1 COMPANY OVERVIEW
31.28.2 REVENUE ANALYSIS
31.28.3 GEOGRAPHIC PRESENCE
31.28.4 PRODUCT PORTFOLIO
31.28.5 RECENT DEVELOPMENTS
31.29 STRIDES PHARMA GLOBAL PTE LTD
31.29.1 COMPANY OVERVIEW
31.29.2 REVENUE ANALYSIS
31.29.3 GEOGRAPHIC PRESENCE
31.29.4 PRODUCT PORTFOLIO
31.29.5 RECENT DEVELOPMENTS
31.3 SYNTHON B.V.
31.30.1 COMPANY OVERVIEW
31.30.2 REVENUE ANALYSIS
31.30.3 GEOGRAPHIC PRESENCE
31.30.4 PRODUCT PORTFOLIO
31.30.5 RECENT DEVELOPMENTS
32 REPORTS
33 CONCLUSION
34 QUESTIONNAIRE
35 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.