Global Ashermans Syndrome Market
Market Size in USD Million
CAGR :
% 
 USD
247.33 Million
USD
412.42 Million
2022
2030
USD
247.33 Million
USD
412.42 Million
2022
2030
| 2023 –2030 | |
| USD 247.33 Million | |
| USD 412.42 Million | |
|
|
|
|
Asherman’s Syndrome Market Analysis and Size
As per the International Asherman's Association, dilatation and curettage (D and C) procedures cause almost 90% of all instances of Asherman syndrome. Following an incomplete miscarriage, a retained placenta after birth, or an elective abortion, a D and C are usually performed. Asherman's Syndrome (AS) is an acquired disorder characterized by intrauterine adhesions (IUA), which produce symptoms such as irregular menstruation, pelvic pain, infertility, recurrent miscarriage, aberrant placentation, and psychological anguish. AS is traditionally thought to be an iatrogenic condition caused by trauma to the pregnant uterus. Different conditions can cause endometrium deterioration, altering the endometrial stem cell niche and resulting in IUAs.
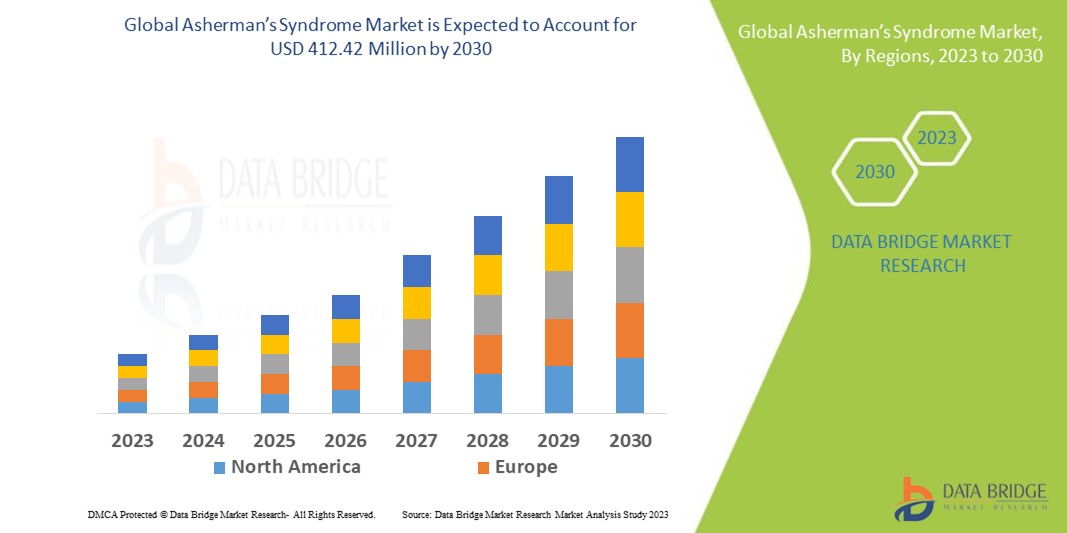
Data Bridge Market Research analyses that the global asherman’s syndrome market which was USD 247.33 million in 2022, would rocket up to USD 412.42 million by 2030, and is expected to undergo a CAGR of 6.6% during the forecast period. This indicates the market value. “Antibiotics” is expected to dominate the treatment segment of the global asherman’s syndrome market owing to the growing demand for better methods for treatment in patients. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Asherman’s Syndrome Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, and Pricing in USD |
|
Segments Covered |
Diagnosis (Hysterosalpingography, Hysterosonogram, Ultrasound, and Others), Treatment (Surgery, Hysteroscopy, Antibiotics, and Others), Dosage Forms (Tablet, Injections, and Others), Route of Administration (Oral, Parenteral, and Others), End-Users (Hospitals, Specialty Clinics, Homecare, and Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, and Others) |
|
Countries Covered |
U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa |
|
Market Players Covered |
Allergan (Ireland), Sanofi (France), Pfizer Inc. (U.S.), GlaxoSmithKline plc (U.K.), Novartis AG (Switzerland), Bayer AG (Germany), Eli Lilly and Company (U.S.), Merck & Co., Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (U.S.), Merck KGaA (Germany), Melinta Therapeutics LLC (U.S.), Basilea Pharmaceutica Ltd. (Switzerland), Tetraphase Pharmaceuticals (U.S.), Paratek Pharmaceuticals, Inc. (U.S.), Sun Pharmaceutical Industries Ltd. (India), Aurobindo Pharma (India), Lupin (India), Teva Pharmaceutical Industries Ltd. (Israel), Abbott (U.S.), among others |
|
Market Opportunities |
|
Market Definition
Asherman's syndrome is a rare, acquired disorder in which scar tissue develops in the uterus or cervix. The uterine walls thicken as a result of the scar tissue, reducing the size of the uterus and, in severe cases, fusing the walls. The adhesions or scar tissue are cut and removed during surgery. Hysteroscopy is frequently used to accomplish this. Small devices and a camera are inserted via the cervix into the uterus. Asherman's syndrome is also termed intrauterine adhesions (IUA).
Global Asherman’s Syndrome Market Dynamics
Drivers
- Rise in the Number of Cases of Asherman’s Syndrome
The surging number of cases of Asherman’s syndrome is a major factor driving the market's growth rate during the forecast period of 2022-2029. Asherman syndrome is a rather uncommon disorder. It mostly affects women who have had many dilation and curettage (D and C) surgeries. Asherman syndrome can also be caused by a severe pelvic infection that is unrelated to surgery. Adhesions in the uterine cavity can also develop following tuberculosis or schistosomiasis infection. In the United States, these infections are uncommon. Even fewer women experience uterine problems as a result of these infections.
- Increasing Investment in Healthcare Infrastructure
Another significant factor influencing the growth rate of Asherman’s syndrome market is the rising healthcare expenditure, which helps in improving its infrastructure. Also, various government organizations aim to improve the healthcare infrastructure by increasing funding, and this will further influence the market dynamics.
- Growing Incidences of Endometriosis
The rising incidences of endometriosis is estimated to enhance the market’s growth rate. Endometriosis, a common inflammatory disease, causes Asherman's syndrome. It's caused by a uterine tissue (endometrium) build-up that can't be shed before menstruation. Lower back or thigh pain, as well as severe pain throughout the menstrual cycle, are possible symptoms.
Opportunities
- Increased Awareness and Diagnosis
Asherman's Syndrome is often underdiagnosed or misdiagnosed, leading to delayed treatment and management. There is an opportunity to raise awareness about the condition among both healthcare professionals and patients. By educating healthcare professionals about the signs, symptoms, and diagnostic approaches for Asherman's Syndrome, more accurate and timely diagnoses can be made. Additionally, creating awareness among women about the condition and its potential impact on fertility and reproductive health can encourage them to seek medical help and receive appropriate treatment.
- Development of Novel Treatment Options
Currently, the mainstay of treatment for Asherman's Syndrome is hysteroscopic surgery to remove adhesions and restore the normal uterine cavity. However, there is a need for additional treatment options, especially for cases with severe adhesions or recurrent disease. Research and development efforts can explore novel therapeutic approaches, such as regenerative medicine techniques, stem cell therapy, and targeted drug delivery systems, to promote tissue regeneration and prevent adhesion recurrence.
Restraints/Challenges
- Underdiagnosis and Misdiagnosis
Asherman's syndrome is often underdiagnosed or misdiagnosed, leading to delayed or ineffective treatment. The lack of awareness among healthcare professionals about the condition and its symptoms can contribute to this challenge. Improving awareness and education among healthcare professionals is crucial to ensure timely and accurate diagnoses.
- Limited Treatment Options
The current treatment for asherman's syndrome primarily involves hysteroscopic surgery to remove adhesions. However, the success rate of this procedure can vary depending on the severity and location of the adhesions. In cases of severe adhesions or recurrent disease, treatment options may be limited, and the chances of achieving a successful pregnancy may be reduced. There is a need for further research and development to explore alternative and more effective treatment options for Asherman's Syndrome.
This global asherman’s syndrome market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the Asherman’s Syndrome market contact Data Bridge Market Research for an analyst brief, our team will help you make an informed market decision to achieve market growth.
Recent Developments
Global Asherman’s Syndrome Market Scope
The global asherman’s syndrome market is segmented on the basis of diagnosis, treatment, dosage forms, route of administration, end-users and distribution channel. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Diagnosis
- Hysterosalpingography
- Hysterosonogram
- Ultrasound
- Others
Treatment
- Surgery
- Hysteroscopy
- Antibiotics
- Others
Dosage Forms
- Tablet
- Injections
- Others
Route of Administration
- Oral
- Parenteral
- Others
End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Global Asherman’s Syndrome Market Regional Analysis/Insights
The global asherman’s syndrome market is analysed, and market size insights and trends are provided by country, diagnosis, treatment, dosage forms, route of administration, end-users and distribution channel as referenced above.
The countries covered in the global asherman’s syndrome market report are U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa.
North America is expected to dominate the global asherman’s syndrome market because of the growing prevalence of asherman’s syndrome, and rising healthcare expenditure will further propel the market’s growth rate in this region. In addition, the growing presence of major key players will further propel the market’s growth rate in this region.
Asia-Pacific is expected to witness significant growth during the forecast period of 2023 to 2030 due to the development of healthcare infrastructure in this region. Also, rising government initiatives will further propel the market’s growth rate in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends, and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of domestic tariffs, and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The global asherman’s syndrome market also provides you with a detailed market analysis for every country's growth in healthcare expenditure for capital equipment, installed base of different kinds of products for the global asherman’s syndrome market, the impact of technology using lifeline curves and changes in healthcare regulatory scenarios and their impact on the global asherman’s syndrome market.
Competitive Landscape and Asherman’s Syndrome Market Share Analysis
The global asherman’s syndrome market competitive landscape provides details by competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width, and breadth, application dominance. The above data points provided are only related to the companies' focus related to the global asherman’s syndrome market.
Some of the major players operating in the global asherman’s syndrome market are:
- Allergan (Ireland)
- Sanofi (France)
- Pfizer Inc. (U.S.)
- GlaxoSmithKline plc (U.K.)
- Novartis AG (Switzerland)
- Bayer AG (Germany)
- Eli Lilly and Company (U.S.)
- Merck & Co., Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Mylan N.V. (U.S.)
- Merck KGaA (Germany)
- Melinta Therapeutics LLC (U.S.)
- Basilea Pharmaceutica Ltd. (Switzerland)
- Tetraphase Pharmaceuticals (U.S.)
- Paratek Pharmaceuticals, Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Aurobindo Pharma (India)
- Lupin (India)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Abbott (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1. INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL ASHERMAN’S SYNDROME MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2. MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL ASHERMAN’S SYNDROME MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL ASHERMAN’S SYNDROME MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3. MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4. EXECUTIVE SUMMARY
5. PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6. INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.1.1 PATENT LANDSCAPE
6.1.2 USPTO NUMBER
6.1.3 PATENT EXPIRY
6.1.4 EPIO NUMBER
6.1.5 PATENT STRENGTH AND QUALITY
6.1.6 PATENT CLAIMS
6.1.7 PATENT CITATIONS
6.1.8 PATENT LITIGATION AND LICENSING
6.1.9 FILE OF PATENT
6.1.10 PATENT RECEIVED CONTRIES
6.1.11 TECHNOLOGY BACKGROUND
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH SPECIALIST
6.8 OTHER KOL SNAPSHOTS
7. EPIDEMIOLOGY
7.1 INCIDENCE OF ALL BY GENDER
7.2 TREATMENT RATE
7.3 MORTALITY RATE
7.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
7.5 PATIENT TREATMENT SUCCESS RATES
8. MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9. REGULATORY FRAMEWORK
9.1 REGULATORY APPROVAL PROCESS
9.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
9.3 REGULATORY APPROVAL PATHWAYS
9.4 LICENSING AND REGISTRATION
9.5 POST-MARKETING SURVEILLANCE
9.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
10. PIPELINE ANALYSIS
10.1 CLINICAL TRIALS AND PHASE ANALYSIS
10.2 DRUG THERAPY PIPELINE
10.3 PHASE III CANDIDATES
10.4 PHASE II CANDIDATES
10.5 PHASE I CANDIDATES
10.6 OTHERS (PRE-CLINICAL AND RESEARCH)
>11. MARKETED DRUG ANALYSIS
11.1 DRUG
11.1.1 BRAND NAME
11.1.2 GENERICS NAME
11.2 THERAPEUTIC INDICTION
11.3 PHARMACOLOGICAL CLASS OF THE DRUG
11.4 DRUG PRIMARY INDICATION
11.5 MARKET STATUS
11.6 MEDICATION TYPE
11.7 DRUG DOSAGES FORM
11.8 DOSAGES AVAILABILITY
11.9 DRUG ROUTE OF ADMINISTRATION
11.10 DOSING FREQUENCY
11.11 DRUG INSIGHT
11.12 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
11.12.1 FORECAST MARKET OUTLOOK
11.12.2 CROSS COMPETITION
11.12.3 THERAPEUTIC PORTFOLIO
11.12.4 CURRENT DEVELOPMENT SCENARIO
12. MARKET ACCESS
12.1 10-YEAR MARKET FORECAST
12.2 CLINICAL TRIAL RECENT UPDATES
12.3 ANNUAL NEW FDA APPROVED DRUGS
12.4 DRUGS MANUFACTURER AND DEALS
12.5 MAJOR DRUG UPTAKE
12.6 CURRENT TREATMENT PRACTICES
12.7 IMPACT OF UPCOMING THERAPY
13. R & D ANALYSIS
13.1 COMPARATIVE ANALYSIS
13.2 DRUG DEVELOPMENTAL LANDSCAPE
13.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
13.4 THERAPEUTIC ASSESSMENT
13.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
14. MARKET OVERVIEW
14.1 DRIVERS
14.2 RESTRAINTS
14.3 OPPORTUNITIES
14.4 CHALLENGES
15. GLOBAL ASHERMAN’S SYNDROME MARKET, BY TREATMENT
15.1 OVERVIEW
15.2 SURGERY
15.2.1 HYSTEROSCOPIC ADHESIOLYSIS
15.2.1.1. MECHANICAL
15.2.1.2. ELECTRICAL
15.2.1.3. THERMAL
15.2.2 LAPAROSCOPIC ADHESIOLYSIS
15.2.3 OTHERS
15.3 NON SURGICAL
15.3.1 PHARMACOLOGICAL THERAPY
15.3.1.1. ANTIBIOTIC
15.3.1.1.1. CIPROFLOXACIN
15.3.1.1.2. DOXYCYCLINE
15.3.1.1.3. CEFIXIME
15.3.1.1.4. OTHERS
15.3.1.2. ESTROGEN TABLETS
15.3.1.2.1. PREMARIN
15.3.1.2.2. PREMPRO
15.3.1.2.3. ACTIVELLA
15.3.1.2.4. OTHERS
15.3.1.3. BIRTH CONTROL PATCHES
15.3.1.4. OTHERS
15.3.2 PHYSICAL THERAPY
15.3.3 OTHERS
15.4 STEM CELLS TREATMENT
16. GLOBAL ASHERMAN’S SYNDROME MARKET, BY PRODUCT TYPE
16.1 OVERVIEW
16.2 INTRAUTERINE DEVICES (IUDS)
16.3 ANTI-ADHESION PRODUCTS
16.4 UTERINE BALLOON STENT
16.5 FOLEY'S CATHETER
16.6 HORMONAL THERAPY PRODUCTS
16.7 OTHERS
17. GLOBAL ASHERMAN’S SYNDROME MARKET, BY DIAGNOSIS
17.1 OVERVIEW
17.2 HYSTEROSALPINGOGRAPHY
17.3 TRANSVAGINAL ULTRASOUND
17.4 HYSTEROSONOGRAM
17.5 HYSTEROSCOPY
17.6 OTHERS
18. GLOBAL ASHERMAN’S SYNDROME MARKET, BY ROUTE OF ADMINISTRATION
18.1 OVERVIEW
18.2 ORAL
18.2.1 TABLET
18.2.2 PILLS/CAPSULES
18.2.3 OTHERS
18.3 PARENTERAL
18.3.1 INTRAVENOUS
18.3.2 TRANSDERMAL
18.3.3 OTHERS
18.4 OTHERS
19. GLOBAL ASHERMAN’S SYNDROME MARKET, BY DRUGS TYPE
19.1 OVERVIEW
19.2 BRANDED
19.3 GENERICS
20. GLOBAL ASHERMAN’S SYNDROME MARKET, BY POPULATION TYPE
20.1 OVERVIEW
20.2 PEDIATRIC
20.2.1 BY SEVERITY LEVEL
20.2.1.1. MILD
20.2.1.2. MODERATE
20.2.1.3. SEVERE
20.2.2 BY CHRONIC LEVEL
20.2.2.1. PRIMARY
20.2.2.2. SECONDARY
20.3 ADULTS
20.3.1 BY SEVERITY LEVEL
20.3.1.1. MILD
20.3.1.2. MODERATE
20.3.1.3. SEVERE
20.3.2 BY CHRONIC LEVEL
20.3.2.1. PRIMARY
20.3.2.2. SECONDARY
20.4 GERIATRIC
20.4.1 BY SEVERITY LEVEL
20.4.1.1. MILD
20.4.1.2. MODERATE
20.4.1.3. SEVERE
20.4.2 BY CHRONIC LEVEL
20.4.2.1. PRIMARY
20.4.2.2. SECONDARY
21. GLOBAL ASHERMAN’S SYNDROME MARKET, BY SEVERITY LEVEL
21.1 OVERVIEW
21.2 MILD
21.3 MODERATE
21.4 SEVERE
22. GLOBAL ASHERMAN’S SYNDROME MARKET, BY PRESCRIPTION TYPE
22.1 OVERVIEW
22.2 PRESCRIPTION DRUGS
22.3 OVER THE COUNTER
23. GLOBAL ASHERMAN’S SYNDROME MARKET, BY END USER
23.1 OVERVIEW
23.2 HOSPITAL
23.2.1 PRIVATE
23.2.2 GOVERNMENT
23.3 SPECIALTY CLINICS
23.4 HOME HEALTHCARE
23.5 AMBULATORY SURGICAL CENTER
23.6 OTHERS
24. GLOBAL ASHERMAN’S SYNDROME MARKET, BY DISTRIBUTION CHANNEL
24.1 OVERVIEW
24.2 DIRECT TENDER
24.3 RETAIL SALES
24.3.1 HOSPITAL PHARMACY
24.3.2 ONLINE PHARMACY
24.3.3 MEDICINE STORES
24.4 OTHERS
25. GLOBAL ASHERMAN’S SYNDROME MARKET, COMPANY LANDSCAPE
25.1 COMPANY SHARE ANALYSIS: GLOBAL
25.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
25.3 COMPANY SHARE ANALYSIS: EUROPE
25.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
25.5 MERGERS & ACQUISITIONS
25.6 NEW PRODUCT DEVELOPMENT & APPROVALS
25.7 EXPANSIONS
25.8 REGULATORY CHANGES
25.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
26. GLOBAL ASHERMAN’S SYNDROME MARKET, BY GEOGRAPHY
GLOBAL ASHERMAN’S SYNDROME MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
26.1 NORTH AMERICA
26.1.1 U.S.
26.1.2 CANADA
26.1.3 MEXICO
26.2 EUROPE
26.2.1 GERMANY
26.2.2 U.K.
26.2.3 ITALY
26.2.4 FRANCE
26.2.5 SPAIN
26.2.6 RUSSIA
26.2.7 SWITZERLAND
26.2.8 TURKEY
26.2.9 BELGIUM
26.2.10 NETHERLANDS
26.2.11 DENMARK
26.2.12 SWEDEN
26.2.13 POLAND
26.2.14 NORWAY
26.2.15 FINLAND
26.2.16 REST OF EUROPE
26.3 ASIA-PACIFIC
26.3.1 JAPAN
26.3.2 CHINA
26.3.3 SOUTH KOREA
26.3.4 INDIA
26.3.5 SINGAPORE
26.3.6 THAILAND
26.3.7 INDONESIA
26.3.8 MALAYSIA
26.3.9 PHILIPPINES
26.3.10 AUSTRALIA
26.3.11 NEW ZEALAND
26.3.12 VIETNAM
26.3.13 TAIWAN
26.3.14 REST OF ASIA-PACIFIC
26.4 SOUTH AMERICA
26.4.1 BRAZIL
26.4.2 ARGENTINA
26.4.3 REST OF SOUTH AMERICA
26.5 MIDDLE EAST AND AFRICA
26.5.1 SOUTH AFRICA
26.5.2 EGYPT
26.5.3 BAHRAIN
26.5.4 UNITED ARAB EMIRATES
26.5.5 KUWAIT
26.5.6 OMAN
26.5.7 QATAR
26.5.8 SAUDI ARABIA
26.5.9 REST OF MIDDLE EAST AND AFRICA
26.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
27. GLOBAL ASHERMAN’S SYNDROME MARKET, SWOT AND DBMR ANALYSIS
28. GLOBAL ASHERMAN’S SYNDROME MARKET, COMPANY PROFILE
28.1 IGENOMIX
28.1.1 COMPANY OVERVIEW
28.1.2 REVENUE ANALYSIS
28.1.3 GEOGRAPHIC PRESENCE
28.1.4 PRODUCT PORTFOLIO
28.1.5 RECENT DEVELOPMENTS
28.2 SUN PHARMACEUTICAL INDUSTRIES, INC.
28.2.1 COMPANY OVERVIEW
28.2.2 REVENUE ANALYSIS
28.2.3 GEOGRAPHIC PRESENCE
28.2.4 PRODUCT PORTFOLIO
28.2.5 RECENT DEVELOPMENTS
28.3 TEVA PHARMACEUTICALS USA, INC.
28.3.1 COMPANY OVERVIEW
28.3.2 REVENUE ANALYSIS
28.3.3 GEOGRAPHIC PRESENCE
28.3.4 PRODUCT PORTFOLIO
28.3.5 RECENT DEVELOPMENTS
28.4 LUPIN PHARMACEUTICALS, INC
28.4.1 COMPANY OVERVIEW
28.4.2 REVENUE ANALYSIS
28.4.3 GEOGRAPHIC PRESENCE
28.4.4 PRODUCT PORTFOLIO
28.4.5 RECENT DEVELOPMENTS
28.5 AUROBINDO PHARMA USA
28.5.1 COMPANY OVERVIEW
28.5.2 REVENUE ANALYSIS
28.5.3 GEOGRAPHIC PRESENCE
28.5.4 PRODUCT PORTFOLIO
28.5.5 RECENT DEVELOPMENTS
28.6 PARATEK PHARMACEUTICALS, INC.
28.6.1 COMPANY OVERVIEW
28.6.2 REVENUE ANALYSIS
28.6.3 GEOGRAPHIC PRESENCE
28.6.4 PRODUCT PORTFOLIO
28.6.5 RECENT DEVELOPMENTS
28.7 TETRAPHASE PHARMACEUTICALS, INC.
28.7.1 COMPANY OVERVIEW
28.7.2 REVENUE ANALYSIS
28.7.3 GEOGRAPHIC PRESENCE
28.7.4 PRODUCT PORTFOLIO
28.7.5 RECENT DEVELOPMENTS
28.8 BASILEA PHARMACEUTICA LTD, ALLSCHWIL
28.8.1 COMPANY OVERVIEW
28.8.2 REVENUE ANALYSIS
28.8.3 GEOGRAPHIC PRESENCE
28.8.4 PRODUCT PORTFOLIO
28.8.5 RECENT DEVELOPMENTS
28.9 MELINTA THERAPEUTICS LLC
28.9.1 COMPANY OVERVIEW
28.9.2 REVENUE ANALYSIS
28.9.3 GEOGRAPHIC PRESENCE
28.9.4 PRODUCT PORTFOLIO
28.9.5 RECENT DEVELOPMENTS
28.10 VIATRIS INC.
28.10.1 COMPANY OVERVIEW
28.10.2 REVENUE ANALYSIS
28.10.3 GEOGRAPHIC PRESENCE
28.10.4 PRODUCT PORTFOLIO
28.10.5 RECENT DEVELOPMENTS
28.11 HIKMA PHARMACEUTICALS PLC
28.11.1 COMPANY OVERVIEW
28.11.2 REVENUE ANALYSIS
28.11.3 GEOGRAPHIC PRESENCE
28.11.4 PRODUCT PORTFOLIO
28.11.5 RECENT DEVELOPMENTS
28.12 ADVACARE PHARMA
28.12.1 COMPANY OVERVIEW
28.12.2 REVENUE ANALYSIS
28.12.3 GEOGRAPHIC PRESENCE
28.12.4 PRODUCT PORTFOLIO
28.12.5 RECENT DEVELOPMENTS
28.13 WELLONA PHARMA
28.13.1 COMPANY OVERVIEW
28.13.2 REVENUE ANALYSIS
28.13.3 GEOGRAPHIC PRESENCE
28.13.4 PRODUCT PORTFOLIO
28.13.5 RECENT DEVELOPMENTS
28.14 BHUMI PHARMACEUTICALS
28.14.1 COMPANY OVERVIEW
28.14.2 REVENUE ANALYSIS
28.14.3 GEOGRAPHIC PRESENCE
28.14.4 PRODUCT PORTFOLIO
28.14.5 RECENT DEVELOPMENTS
28.15 ADEN HEALTHCARE
28.15.1 COMPANY OVERVIEW
28.15.2 REVENUE ANALYSIS
28.15.3 GEOGRAPHIC PRESENCE
28.15.4 PRODUCT PORTFOLIO
28.15.5 RECENT DEVELOPMENTS
28.16 JACKSON LABORATORIES PVT LTD
28.16.1 COMPANY OVERVIEW
28.16.2 REVENUE ANALYSIS
28.16.3 GEOGRAPHIC PRESENCE
28.16.4 PRODUCT PORTFOLIO
28.16.5 RECENT DEVELOPMENTS
28.17 ZOIC BIOTECH PRIVATE LIMITED
28.17.1 COMPANY OVERVIEW
28.17.2 REVENUE ANALYSIS
28.17.3 GEOGRAPHIC PRESENCE
28.17.4 PRODUCT PORTFOLIO
28.17.5 RECENT DEVELOPMENTS
28.18 F. HOFFMANN-LA ROCHE LTD
28.18.1 COMPANY OVERVIEW
28.18.2 REVENUE ANALYSIS
28.18.3 GEOGRAPHIC PRESENCE
28.18.4 PRODUCT PORTFOLIO
28.18.5 RECENT DEVELOPMENTS
28.19 ANI PHARMACEUTICALS, INC.
28.19.1 COMPANY OVERVIEW
28.19.2 REVENUE ANALYSIS
28.19.3 GEOGRAPHIC PRESENCE
28.19.4 PRODUCT PORTFOLIO
28.19.5 RECENT DEVELOPMENTS
28.20 PFIZER INC.
28.20.1 COMPANY OVERVIEW
28.20.2 REVENUE ANALYSIS
28.20.3 GEOGRAPHIC PRESENCE
28.20.4 PRODUCT PORTFOLIO
28.20.5 RECENT DEVELOPMENTS
28.21 SANOFI
28.21.1 COMPANY OVERVIEW
28.21.2 REVENUE ANALYSIS
28.21.3 GEOGRAPHIC PRESENCE
28.21.4 PRODUCT PORTFOLIO
28.21.5 RECENT DEVELOPMENTS
28.22 GSK PLC.
28.22.1 COMPANY OVERVIEW
28.22.2 REVENUE ANALYSIS
28.22.3 GEOGRAPHIC PRESENCE
28.22.4 PRODUCT PORTFOLIO
28.22.5 RECENT DEVELOPMENTS
28.23 NOVARTIS AG
28.23.1 COMPANY OVERVIEW
28.23.2 REVENUE ANALYSIS
28.23.3 GEOGRAPHIC PRESENCE
28.23.4 PRODUCT PORTFOLIO
28.23.5 RECENT DEVELOPMENTS
28.24 DELMONT IMAGING
28.24.1 COMPANY OVERVIEW
28.24.2 REVENUE ANALYSIS
28.24.3 GEOGRAPHIC PRESENCE
28.24.4 PRODUCT PORTFOLIO
28.24.5 RECENT DEVELOPMENTS
28.25 STERIMED GROUP
28.25.1 COMPANY OVERVIEW
28.25.2 REVENUE ANALYSIS
28.25.3 GEOGRAPHIC PRESENCE
28.25.4 PRODUCT PORTFOLIO
28.25.5 RECENT DEVELOPMENTS
28.26 SCW MEDICATH LTD
28.26.1 COMPANY OVERVIEW
28.26.2 REVENUE ANALYSIS
28.26.3 GEOGRAPHIC PRESENCE
28.26.4 PRODUCT PORTFOLIO
28.26.5 RECENT DEVELOPMENTS
28.27 ABBOTT
28.27.1 COMPANY OVERVIEW
28.27.2 REVENUE ANALYSIS
28.27.3 GEOGRAPHIC PRESENCE
28.27.4 PRODUCT PORTFOLIO
28.27.5 RECENT DEVELOPMENTS
28.28 ABBVIE INC.
28.28.1 COMPANY OVERVIEW
28.28.2 REVENUE ANALYSIS
28.28.3 GEOGRAPHIC PRESENCE
28.28.4 PRODUCT PORTFOLIO
28.28.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
29. RELATED REPORTS
30. CONCLUSION
31. QUESTIONNAIRE
32. ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.