Global Bevacizumab Market
Market Size in USD Million
CAGR :
% 
 USD
5,561.07 Million
USD
8,560.41 Million
2022
2030
USD
5,561.07 Million
USD
8,560.41 Million
2022
2030
| 2023 –2030 | |
| USD 5,561.07 Million | |
| USD 8,560.41 Million | |
|
|
|
|
Bevacizumab Market Analysis and Size
The increasing prevalence of cancer and changing lifestyle along with support groups are being instrumental for cancer patients in coping with cancer by providing funds and supportive care will boost the market growth. Emerging markets and the collaboration of global pharmaceutical manufacturers also have a positive impact on the market.
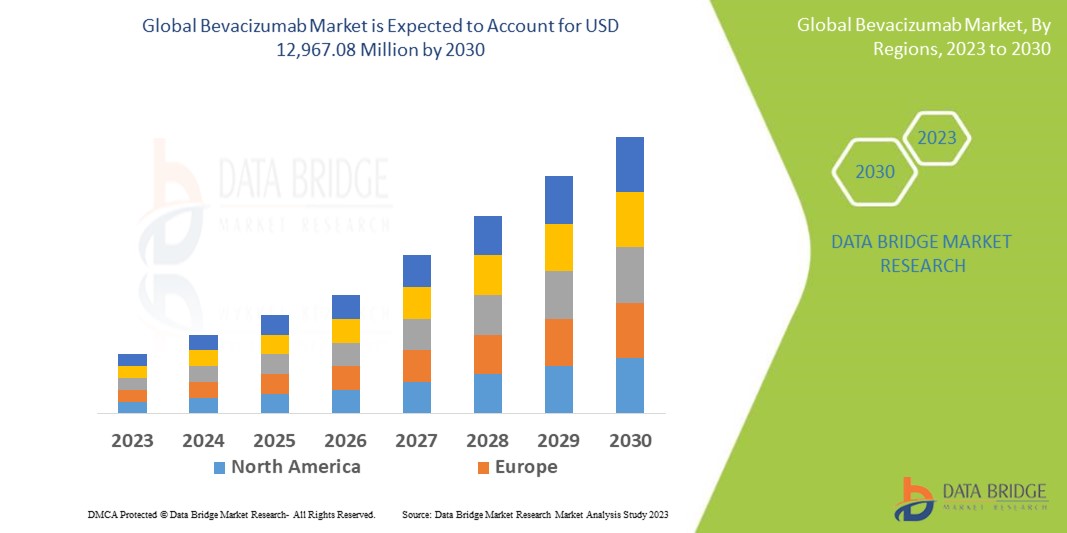
Data Bridge Market Research analyses that the market, which was USD 6,679.14 million in 2022, would rocket up to USD 12,967.08 million by 2030 and is expected to undergo a CAGR of 9.20% during the forecast period. “Cancer” dominates the disease segment of the bevacizumab market owing to the growing demand for bevacizumab to treat cancer. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Bevacizumab Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021(Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
By Diseases (Cancer and Eye Disorder), Brand Name (Avastin, Mvasi, Zirabev, and Versavo), Dosage (100mg and 400mg), Application (Non Squamous Non-Small Cell Lung Cancer, Recurrent Glioblastoma, Cervical Cancer, Colorectal Cell Cancer, Ovarian Cancer, Proliferative Diabetic Retinopathy, Malignant Glioma, Neurofibromatosis, Pancreatic Cancer, and Others), End-Users (Hospitals, Cancer Supportive Centres, Home Healthcare, Academic and Research Institutes, and Others), Distribution Channel (Direct Tender, Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, and Others) |
|
Countries Covered |
U.S., Canada, and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E, South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina and rest of South America |
|
Market Players Covered |
Genentech (U.S.), Amgen (U.S.), Pfizer Inc. (U.S.), Allergan (Ireland), Biocon (India), Reliance Life Sciences (India), Beacon Pharmaceuticals Limited (Bangladesh), Celgene Corporation (U.S.), Mylan Inc, (U.S.), Dr. Reddy’s Laboratories Ltd (India), Eli Lilly and Company (India), BioXpress Therapeutics (Switzerland), Teva Pharmaceutical industries Ltd. (Isarel), CELLTRION INC. (South Korea), mAbxience (Switzerland), Samsung Bioepis (China), FUJIFILM KYOWA KIRIN BIOLOGICS Co., Ltd. (Japan), Hetero Drugs (U.S.), AryoGen Pharmed (Iran) and Zydus Cadila (India) |
|
Market Opportunities |
|
Market Definition
Bevacizumab is a humanized recombinant monoclonal antibody that is effective against vascular endothelial growth factor called VEGF (pro angiogenic cytokine), which is made up of cells that help in new blood vessel formation. Bevacizumab acts by binding to VEGF and subsequently inhibiting its receptor binding process, which prevents the growth of the tumor and cancerous cells. Bevacizumab is currently undergoing many trials for being combined with other drugs for cancer treatment.
Global Bevacizumab Market Dynamics
Drivers
- Increasing cancer prevalence
Cancer remains a major global health problem, and the incidence of various types of cancer is increasing. Bevacizumab is the main treatment for many types of cancer, including colon, lung, breast, and kidney cancer. The increasing prevalence of cancer worldwide increases the demand for bevacizumab as an effective treatment option, driving market growth.
- Advancements in biotechnology and research
The field of biotechnology and cancer research is rapidly evolving, leading to the discovery of new therapeutic targets and treatments. Bevacizumab, an anti-angiogenic drug, has shown efficacy in inhibiting tumor growth by targeting blood vessel formation. Continued R&D efforts to find new applications and combinations for bevacizumab will drive market growth.
- Growing investment in healthcare facilities
Surging focus on improving the condition of healthcare facilities and improving the overall healthcare infrastructure is another important factor fostering the growth of the market. The rising number of partnerships and strategic collaborations between the public and private players about funding and application of new and improved technology further creates lucrative market opportunities.
- Technological advancements in drug delivery systems
The development of improved drug delivery systems has improved the delivery and efficacy of bevacizumab. For example, the introduction of intravitreal injections in treating eye diseases facilitated the use of bevacizumab in conditions such as macular degeneration and diabetic retinopathy. Technological advancements in drug delivery systems improve patient comfort and enable targeted deliveries, thus driving market growth.
Opportunities
- Growing demand in emerging markets
Emerging markets present a significant opportunity for the bevacizumab market. As healthcare infrastructure and access to advanced treatments improve in these regions, the demand for bevacizumab is expected to rise. Emerging economies, such as China, India, Brazil, and others, have a large population base and increasing incidences of cancer. By strategically entering these markets and addressing affordability challenges, companies can tap into the growing demand and expand their market share.
- Expansion into new indications
Although bevacizumab is mainly used in treating various cancers, it can be expanded to new indications. Bevacizumab is currently being studied for its effectiveness in other diseases, such as age-related macular degeneration, diabetic retinopathy, and certain neurological diseases. Successfully expanding the range of indications for bevacizumab could open new market segments and stimulate revenue.
Challenges/ Restraints
- Patent expiry and potential generic competition
Bevacizumab has limited patent exclusivity, and if the patents expire, it opens the door to potential generic competition. Generic versions of bevacizumab, also known as biosimilars, may enter the market at lower prices, challenging incumbent drugmakers for market share and profitability. Successfully navigating the competitive environment by introducing biosimilars is a major challenge for companies in the bevacizumab market.
- High cost and limited affordability
Bevacizumab is a complex biological drug with high production costs. As a result, medicines are expensive, making them unaffordable for a large part of the population, especially in developing and low-income countries. The high cost of bevacizumab may limit its availability and adoption, thus hindering market growth.
- Stringent regulatory requirements
The regulatory processes and requirements for approval of bevacizumab and other biologics can be rigorous and time-consuming. Regulatory approval from different authorities in multiple countries can be complex and expensive. Regulatory challenges may delay market entry and limit the availability of bevacizumab, affecting the growth potential of this market.
The global Bevacizumab market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the Bevacizumab market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Development
-
In 2020, the European Medicines Agency (EMA) approved the first biosimilar of bevacizumab for the treatment of multiple types of cancer. The availability of biosimilars contributes to increased competition in the market and has the potential to enhance patient access to bevacizumab therapy.
-
In 2020, the U.S. Food and Drug Administration (FDA) approved bevacizumab in combination with chemotherapy as a treatment option for patients with advanced hepatocellular carcinoma (liver cancer). Ongoing clinical trials and research also evaluate the use of bevacizumab in combination with other therapies and its potential in different types of cancers and non-cancerous conditions.
Global Bevacizumab Market Scope
This market is segmented on the basis of diseases, brand name, Dosage, application, end-users, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Diseases
- Cancer
- Eye diseases
Brand Name
- Avastin
- Mvasi
- Zirabev
- Others
Dosage
- 100 mg
- 400 mg
Application
- Non squamous non-small cell lung cancer
- Recurrent glioblastoma
- Cervical cancer
- Colorectal cell cancer
- Ovarian cancer
- Proliferative diabetic retinopathy
- Malignant glioma
- Neurofibromatosis
- Pancreatic cancer
- Others
End User
- Hospitals
- Cancer supportive centres
- Home healthcare
- Academic and research institutes
- Others
Distribution channel
- Direct tender
- Hospital pharmacy
- Retail pharmacy
- Online pharmacy
- Others
Global Bevacizumab Market Regional Analysis/Insights
The global bevacizumab market is analysed, and market size insights and trends are provided by country, diseases, brand name, Dosage, application, end-users, and distribution channel as referenced above.
The countries covered in this market report are U.S., Canada, and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E, South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina and rest of South America.
Europe accounts for the largest market share due to the increased consumption of bevacizumab to treat various types of cancer.
Asia-Pacific is expected to account for the largest market share over the coming years for the market due to increasing prevalence and growing healthcare expenditure.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure growth Installed base and New Technology Penetration
The market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for Bevacizumab market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the market. The data is available for historic period 2010-2020.
Competitive Landscape and Global Bevacizumab Market Share Analysis
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Some of the major players operating in the market are:
- Genentech (U.S.)
- Amgen (U.S.)
- Pfizer Inc. (U.S.)
- Allergan (Ireland)
- Biocon (India)
- Reliance Life Sciences (India)
- Beacon Pharmaceuticals Limited (Bangladesh)
- Celgene Corporation (U.S.)
- Mylan Inc, (U.S.)
- Dr. Reddy’s Laboratories Ltd (India)
- Eli Lilly and Company (India)
- BioXpress Therapeutics (Switzerland)
- Teva Pharmaceutical industries Ltd. (Isarel)
- CELLTRION INC. (South Korea)
- mAbxience (Switzerland)
- Samsung Bioepis (China)
- FUJIFILM KYOWA KIRIN BIOLOGICS Co., Ltd. (Japan)
- Hetero Drugs (U.S.)
- AryoGen Pharmed (Iran)
- Zydus Cadila (India)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL BEVACIZUMAB MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL BEVACIZUMAB MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY BASED MODELLING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL BEVACIZUMAB MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.1.1 PATENT LANDSCAPE
6.1.2 USPTO NUMBER
6.1.3 PATENT EXPIRY
6.1.4 EPIO NUMBER
6.1.5 PATENT STRENGTH AND QUALITY
6.1.6 PATENT CLAIMS
6.1.7 PATENT CITATIONS
6.1.8 PATENT LITIGATION AND LICENSING
6.1.9 FILE OF PATENT
6.1.10 PATENT RECEIVED CONTRIES
6.1.11 TECHNOLOGY BACKGROUND
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH SPECIALIST
6.8 OTHER KOL SNAPSHOTS
7 EPIDEMIOLOGY
7.1 INCIDENCE OF ALL BY GENDER
7.2 TREATMENT RATE
7.3 MORTALITY RATE
7.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
7.5 PATIENT TREATMENT SUCCESS RATES
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 REGULATORY FRAMEWORK
9.1 REGULATORY APPROVAL PROCESS
9.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
9.3 REGULATORY APPROVAL PATHWAYS
9.4 LICENSING AND REGISTRATION
9.5 POST-MARKETING SURVEILLANCE
9.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
10 PIPELINE ANALYSIS
10.1 CLINICAL TRIALS AND PHASE ANALYSIS
10.2 DRUG THERAPY PIPELINE
10.3 PHASE III CANDIDATES
10.4 PHASE II CANDIDATES
10.5 PHASE I CANDIDATES
10.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR XX
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
11 MARKETED DRUG ANALYSIS
11.1 DRUG
11.1.1 BRAND NAME
11.1.2 GENERICS NAME
11.2 THERAPEUTIC INDICTION
11.3 PHARMACOLOGICAL CLASS OF THE DRUG
11.4 DRUG PRIMARY INDICATION
11.5 MARKET STATUS
11.6 MEDICATION TYPE
11.7 DRUG DOSAGES FORM
11.8 DOSAGES AVAILABILITY
11.9 DRUG ROUTE OF ADMINISTRATION
11.1 DOSING FREQUENCY
11.11 DRUG INSIGHT
11.12 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
11.12.1 FORECAST MARKET OUTLOOK
11.12.2 CROSS COMPETITION
11.12.3 THERAPEUTIC PORTFOLIO
11.12.4 CURRENT DEVELOPMENT SCENARIO
12 MARKET ACCESS
12.1 10-YEAR MARKET FORECAST
12.2 CLINICAL TRIAL RECENT UPDATES
12.3 ANNUAL NEW FDA APPROVED DRUGS
12.4 DRUGS MANUFACTURER AND DEALS
12.5 MAJOR DRUG UPTAKE
12.6 CURRENT TREATMENT PRACTICES
12.7 IMPACT OF UPCOMING THERAPY
13 R & D ANALYSIS
13.1 COMPARATIVE ANALYSIS
13.2 DRUG DEVELOPMENTAL LANDSCAPE
13.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
13.4 THERAPEUTIC ASSESSMENT
13.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
14 MARKET OVERVIEW
14.1 DRIVERS
14.2 RESTRAINTS
14.3 OPPORTUNITIES
14.4 CHALLENGES
15 GLOBAL BEVACIZUMAB MARKET, BY DISEASE
15.1 OVERVIEW
15.2 CANCER
15.2.1 NON SQUAMOUS NON-SMALL CELL LUNG CANCER
15.2.2 RECURRENT GLIOBLASTOMA
15.2.3 CERVICAL CANCER
15.2.4 RENAL CELL CARCINOMA:
15.2.5 COLORECTAL CELL CANCER
15.2.6 OVARIAN CANCER
15.2.7 PANCREATIC CANCER
15.2.8 OTHERS
15.3 EYE DISORDER
15.3.1 AGE-RELATED MACULAR DEGENERATION (AMD)
15.3.2 DIABETIC RETINOPATHY
15.3.3 OTHERS
16 GLOBAL BEVACIZUMAB MARKET, BY DRUGS
16.1 OVERVIEW
16.2 MARKETED/BIOLOGICS DRUGS
16.3 BIOSIMILAR
17 GLOBAL BEVACIZUMAB MARKET, BY BRANDS
17.1 OVERVIEW
17.2 AVASTIN
17.3 ZIRABEV
17.4 MVASI
17.5 VERSAVO
17.6 OTHERS
18 GLOBAL BEVACIZUMAB MARKET, BY DOSAGE STRENGTH
18.1 OVERVIEW
18.2 100 MG
18.3 400 MG
18.4 OTHERS
19 GLOBAL BEVACIZUMAB MARKET, BY APPLICATIONS
19.1 OVERVIEW
19.2 BIOMEDICINE
19.3 NANOTECHNOLOGY
19.4 NEURO-ONCOLOGY
20 GLOBAL BEVACIZUMAB MARKET, BY END USER
20.1 OVERVIEW
20.2 HOSPITALS
20.3 CANCER SUPPORTIVE CENTRES
20.4 HOME HEALTHCARE
20.5 ACADEMIC AND RESEARCH INSTITUTES
20.6 OTHERS
21 GLOBAL BEVACIZUMAB MARKET, BY DISTRIBUTION CHANNEL
21.1 OVERVIEW
21.2 DIRECT TENDER
21.3 HOSPITAL PHARMACY
21.4 RETAIL PHARMACY
21.5 ONLINE PHARMACY
21.6 OTHERS
22 GLOBAL BEVACIZUMAB MARKET, COMPANY LANDSCAPE
22.1 COMPANY SHARE ANALYSIS: GLOBAL
22.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
22.3 COMPANY SHARE ANALYSIS: EUROPE
22.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
22.5 MERGERS & ACQUISITIONS
22.6 NEW PRODUCT DEVELOPMENT & APPROVALS
22.7 EXPANSIONS
22.8 REGULATORY CHANGES
22.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
23 GLOBAL BEVACIZUMAB MARKET, BY GEOGRAPHY
GLOBAL BEVACIZUMAB MARKET (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
23.1 NORTH AMERICA
23.1.1 U.S.
23.1.2 CANADA
23.1.3 MEXICO
23.2 EUROPE
23.2.1 GERMANY
23.2.2 FRANCE
23.2.3 U.K.
23.2.4 HUNGARY
23.2.5 LITHUANIA
23.2.6 AUSTRIA
23.2.7 IRELAND
23.2.8 NORWAY
23.2.9 POLAND
23.2.10 ITALY
23.2.11 SPAIN
23.2.12 RUSSIA
23.2.13 TURKEY
23.2.14 NETHERLANDS
23.2.15 SWITZERLAND
23.2.16 REST OF EUROPE
23.3 ASIA-PACIFIC
23.3.1 JAPAN
23.3.2 CHINA
23.3.3 SOUTH KOREA
23.3.4 INDIA
23.3.5 AUSTRALIA
23.3.6 SINGAPORE
23.3.7 THAILAND
23.3.8 MALAYSIA
23.3.9 INDONESIA
23.3.10 PHILIPPINES
23.3.11 VIETNAM
23.3.12 REST OF ASIA-PACIFIC
23.4 SOUTH AMERICA
23.4.1 BRAZIL
23.4.2 ARGENTINA
23.4.3 PERU
23.4.4 REST OF SOUTH AMERICA
23.5 MIDDLE EAST AND AFRICA
23.5.1 SOUTH AFRICA
23.5.2 SAUDI ARABIA
23.5.3 UAE
23.5.4 EGYPT
23.5.5 KUWAIT
23.5.6 ISRAEL
23.5.7 REST OF MIDDLE EAST AND AFRICA
23.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
24 GLOBAL BEVACIZUMAB MARKET, SWOT AND DBMR ANALYSIS
25 GLOBAL BEVACIZUMAB MARKET, COMPANY PROFILE
25.1 AMGEN, INC
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPMENTS
25.2 PFIZER INC.
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPMENTS
25.3 GENENTECH, INC. (A MEMBER OF THE ROCHE GROUP )
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPMENTS
25.4 HETERO HEALTHCARE LIMITED.
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPMENTS
25.5 VIATRIS INC. AND BIOCON BIOLOGICS LTD.
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPMENTS
25.6 AMNEAL PHARMACEUTICALS, INC AND MABXIENCE
25.6.1 COMPANY OVERVIEW
25.6.2 REVENUE ANALYSIS
25.6.3 GEOGRAPHIC PRESENCE
25.6.4 PRODUCT PORTFOLIO
25.6.5 RECENT DEVELOPMENTS
25.7 RPG LIFE SCIENCES LIMITED
25.7.1 COMPANY OVERVIEW
25.7.2 REVENUE ANALYSIS
25.7.3 GEOGRAPHIC PRESENCE
25.7.4 PRODUCT PORTFOLIO
25.7.5 RECENT DEVELOPMENTS
25.8 FRESENIUS KABI AG
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPMENTS
25.9 OUTLOOK THERAPEUTICS, INC.
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPMENTS
25.1 RELIANCE LIFE SCIENCES
25.10.1 COMPANY OVERVIEW
25.10.2 REVENUE ANALYSIS
25.10.3 GEOGRAPHIC PRESENCE
25.10.4 PRODUCT PORTFOLIO
25.10.5 RECENT DEVELOPMENTS
25.11 BEACON PHARMACEUTICALS PLC
25.11.1 COMPANY OVERVIEW
25.11.2 REVENUE ANALYSIS
25.11.3 GEOGRAPHIC PRESENCE
25.11.4 PRODUCT PORTFOLIO
25.11.5 RECENT DEVELOPMENTS
25.12 BIO-THERA SOLUTIONS, LTD
25.12.1 COMPANY OVERVIEW
25.12.2 REVENUE ANALYSIS
25.12.3 GEOGRAPHIC PRESENCE
25.12.4 PRODUCT PORTFOLIO
25.12.5 RECENT DEVELOPMENTS
25.13 SAMSUNG BIOEPIS NL B.V.
25.13.1 COMPANY OVERVIEW
25.13.2 REVENUE ANALYSIS
25.13.3 GEOGRAPHIC PRESENCE
25.13.4 PRODUCT PORTFOLIO
25.13.5 RECENT DEVELOPMENTS
25.14 BIOXPRESS THERAPEUTICS SA
25.14.1 COMPANY OVERVIEW
25.14.2 REVENUE ANALYSIS
25.14.3 GEOGRAPHIC PRESENCE
25.14.4 PRODUCT PORTFOLIO
25.14.5 RECENT DEVELOPMENTS
25.15 DR. REDDY’S LABORATORIES LTD.
25.15.1 COMPANY OVERVIEW
25.15.2 REVENUE ANALYSIS
25.15.3 GEOGRAPHIC PRESENCE
25.15.4 PRODUCT PORTFOLIO
25.15.5 RECENT DEVELOPMENTS
25.16 ARYOGEN PHARMED
25.16.1 COMPANY OVERVIEW
25.16.2 REVENUE ANALYSIS
25.16.3 GEOGRAPHIC PRESENCE
25.16.4 PRODUCT PORTFOLIO
25.16.5 RECENT DEVELOPMENTS
25.17 ZYDUS CADILA HEALTHCARE LTD
25.17.1 COMPANY OVERVIEW
25.17.2 REVENUE ANALYSIS
25.17.3 GEOGRAPHIC PRESENCE
25.17.4 PRODUCT PORTFOLIO
25.17.5 RECENT DEVELOPMENTS
25.18 HETERO HEALTHCARE LIMITED.
25.18.1 COMPANY OVERVIEW
25.18.2 REVENUE ANALYSIS
25.18.3 GEOGRAPHIC PRESENCE
25.18.4 PRODUCT PORTFOLIO
25.18.5 RECENT DEVELOPMENTS
25.19 ARYOGEN PHARMED
25.19.1 COMPANY OVERVIEW
25.19.2 REVENUE ANALYSIS
25.19.3 GEOGRAPHIC PRESENCE
25.19.4 PRODUCT PORTFOLIO
25.19.5 RECENT DEVELOPMENTS
25.2 CELLTRION INC.
25.20.1 COMPANY OVERVIEW
25.20.2 REVENUE ANALYSIS
25.20.3 GEOGRAPHIC PRESENCE
25.20.4 PRODUCT PORTFOLIO
25.20.5 RECENT DEVELOPMENTS
25.21 INNOVENT AND ELI LILLY AND COMPANY
25.21.1 COMPANY OVERVIEW
25.21.2 REVENUE ANALYSIS
25.21.3 GEOGRAPHIC PRESENCE
25.21.4 PRODUCT PORTFOLIO
25.21.5 RECENT DEVELOPMENTS
25.22 BIOCAD
25.22.1 COMPANY OVERVIEW
25.22.2 REVENUE ANALYSIS
25.22.3 GEOGRAPHIC PRESENCE
25.22.4 PRODUCT PORTFOLIO
25.22.5 RECENT DEVELOPMENTS
25.23 TANVEX BIOPHARMA INC.
25.23.1 COMPANY OVERVIEW
25.23.2 REVENUE ANALYSIS
25.23.3 GEOGRAPHIC PRESENCE
25.23.4 PRODUCT PORTFOLIO
25.23.5 RECENT DEVELOPMENTS
25.24 SHANGHAI HENLIUS BIOTECH, INC
25.24.1 COMPANY OVERVIEW
25.24.2 REVENUE ANALYSIS
25.24.3 GEOGRAPHIC PRESENCE
25.24.4 PRODUCT PORTFOLIO
25.24.5 RECENT DEVELOPMENTS
25.25 PRESTIGE BIOPHARMA LTD.
25.25.1 COMPANY OVERVIEW
25.25.2 REVENUE ANALYSIS
25.25.3 GEOGRAPHIC PRESENCE
25.25.4 PRODUCT PORTFOLIO
25.25.5 RECENT DEVELOPMENTS
25.26 CENTUS BIOTHERAPEUTICS LTD., A JOINT VENTURE BETWEEN FUJIFILM KYOWA KIRIN BIOLOGICS CO., LTD. AND ASTRAZENECA PLC
25.26.1 COMPANY OVERVIEW
25.26.2 REVENUE ANALYSIS
25.26.3 GEOGRAPHIC PRESENCE
25.26.4 PRODUCT PORTFOLIO
25.26.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
26 RELATED REPORTS
27 CONCLUSION
28 QUESTIONNAIRE
29 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.