Global Bovine Ephemeral Fever Vaccines Market
Market Size in USD Million
CAGR :
% 
 USD
70.34 Million
USD
90.49 Million
2022
2030
USD
70.34 Million
USD
90.49 Million
2022
2030
| 2023 –2030 | |
| USD 70.34 Million | |
| USD 90.49 Million | |
|
|
|
|
Bovine Ephemeral Fever Vaccines Market Analysis and Size
Bovine ephemeral fever is a type of disease of cattle and water buffalo which is caused by a rhabdovirus and is transmitted through biting insects and flying. Due to the inflammatory nature of the disease, NSAIDs are very efficient at relieving clinical signs and pain. The rapidly increasing demand from pharmaceutical companies is amongst the important factors intensifying the growth and demand of global bovine ephemeral fever vaccines market. In addition, the rising geriatric inhabitant across the globe is also contributing to the growth in the global market over the forecast period of 2023 to 20230.
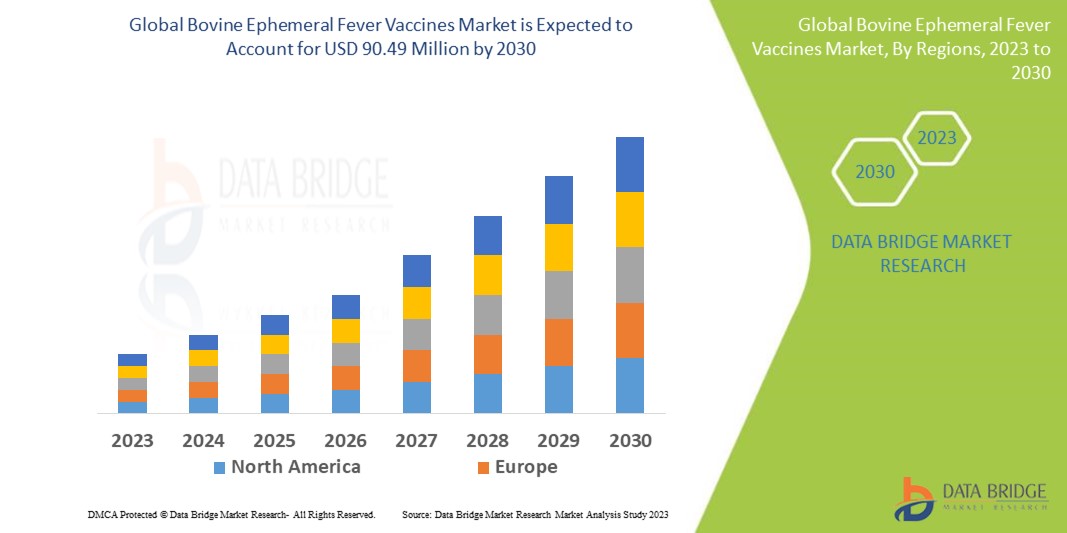
Data Bridge Market Research analyses that the global bovine ephemeral fever vaccines market was valued at USD 70.34 million in 2022 and is further estimated to reach USD 90.49 million by 2030, and is expected to grow at a CAGR of 3.2% during the forecast period of 2023 to 2030. The market report curated by the Data Bridge Market Research team includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Bovine Ephemeral Fever Vaccines Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 – 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Vaccine Type (Live Vaccine, Killed Vaccine, Others), Application (Cattle, Water Buffaloes), Application (Cattle, Water Buffaloes), End Users (Medical Device Companies, Pharmaceutical industries, Animal Hospitals, Other End Users) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Peru, Brazil, Argentina and Rest of South America as part of South America, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Hungary, Lithuania, Austria, Ireland, Norway, Poland, Rest of Europe in Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Vietnam, Rest of Asia-Pacific (APAC) in Asia-Pacific (APAC), South Africa, Saudi Arabia, U.A.E., Kuwait, Israel, Egypt, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA) |
|
Market Players Covered |
Zoetis Services LLC (U.S.), Nutri Pharmax Sdn Bhd (Malaysia), Octavoscene (Pty) Ltd (South Africa), Kyoto Biken Laboratories, Inc. (Japan), Indian Immunologicals Ltd. (India) and CAVAC Network (France) |
|
Market Opportunities |
|
Market Definition
The global bovine ephemeral fever vaccine market refers to the market for vaccines specifically designed for the prevention and control of global bovine ephemeral fever worldwide. Bovine ephemeral fever, also known as three-day disease, is a viral disease that mainly affects cattle and sometimes water buffalo. It is caused by the bovine ephemeral fever virus, which is spread by insect vectors, especially mosquitoes.
Global bovine ephemeral fever vaccine market includes the production, distribution and sale of vaccines immunizing. These vaccines are developed using various techniques, including inactivated vaccines, live attenuated vaccines and combination vaccines. They are designed to stimulate the immune response of cattle, develop immunity against global bovine ephemeral fever and reduce the severity and duration of the disease.
Bovine Ephemeral Fever Vaccines Market Dynamics
Drivers
- Increasing Prevalence of Bovine Ephemeral Fever
Rising incidence of short-lived bovine fever, a viral disease of cattle, is a major driving factor for the global short-lived bovine fever vaccine market. As the disease can cause huge economic losses to the livestock industry, there is a growing demand for effective vaccines to prevent its spread. This impetus is expected to boost the market growth of short-lived bovine fever vaccine.
- Growing Awareness and Government Initiatives
Growing awareness among ranchers and governments about the importance of vaccination to combat short-lived bovine fever has increased the demand for vaccines. Governments around the world have implemented vaccination programs and efforts to limit the impact of the disease on cattle populations. It is hoped that these efforts will facilitate the introduction of a short-lived bovine fever vaccine.
- Advancements in Vaccine Technology
Technological advances in vaccine development have produced more efficient and safer short-lived bovine fever vaccines. New vaccine formulations, improved delivery methods, and enhanced immune responses are having a positive impact on the market. The availability of advanced vaccines has facilitated the introduction and use of short-acting bovine fever vaccines.
Opportunities
- Untapped Regional Markets
There are some untapped regional markets with large cattle populations that have not yet introduced a short-lived bovine fever vaccine on a large scale. Expanding market reach into these regions represents significant growth opportunities for vaccine makers, and targeting these untapped markets could lead to increased sales and market expansion.
- Upsurge in R and D for New Vaccine Candidates
Research and development (R and D) efforts focused on developing new, more effective, short-lived bovine fever vaccines could create lucrative opportunities in the market. Investing in research and development of new vaccine candidates with improved efficacy, safety, and long-term persistence from disease can attract significant attention and market share.
Restraints/ Challenges
- Lack of Awareness and Infrastructure in Developing Regions
A major barrier to the global short-lived bovine fever vaccine market is lack of awareness and infrastructure in some developing regions. In these areas, farmers may not have access to good information about diseases and vaccine availability. Moreover, lack of cold chain facilities for storage and distribution of vaccines poses a challenge to the market growth in these regions.
- Limited Research Data and Knowledge
A challenge for the global short-lived global bovine fever vaccine market is the limited availability of comprehensive research data and knowledge on this disease. Further research and data collection on epidemiology, vaccine efficacy and long-term immunity are needed to better understand and improve vaccine development. Lack of solid data may hinder the potential growth of the market and limit the progress of the short-lived bovine fever vaccine.
This global bovine ephemeral fever vaccines market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global bovine ephemeral fever vaccines market Contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In October 2019, Zoetis launched "Bovilis Bovine Ephemeral Fever," a live attenuated vaccine for the prevention of bovine ephemeral fever in cattle
- In November 2018, Zoetis acquired Abaxis, a leading global provider of veterinary diagnostic instruments, to strengthen its presence in the animal health diagnostics market
Global Bovine Ephemeral Fever Vaccines Market Scope
The bovine ephemeral fever vaccines market is segmented on the basis of vaccine type, application and end users. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Vaccine Type
- Live Vaccine
- Killed Vaccine
- Others
Application
- Cattle
- Water Buffaloes
End Users
- Medical Device Companies
- Pharmaceutical Industries
- Animal Hospitals
- Other End Users
Global Bovine Ephemeral Fever Vaccines Market Regional Analysis/Insights
The global bovine ephemeral fever vaccines market is analyzed and market size insights and trends are provided by country, vaccine type, application and end users as referenced above.
The countries covered in the Global Bovine Ephemeral Fever Vaccines Market report are the U.S., Canada and Mexico in North America, Peru, Brazil, Argentina and Rest of South America as part of South America, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Hungary, Lithuania, Austria, Ireland, Norway, Poland, Rest of Europe in Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Vietnam, Rest of Asia-Pacific (APAC) in Asia-Pacific (APAC), South Africa, Saudi Arabia, U.A.E, Kuwait, Israel, Egypt, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA)
North America dominates the global bovine ephemeral fever vaccines market due to the new product launches and early treatment adoption within the region.
Asia-Pacific is expected to witness the fastest growth during the forecast period 2023-2030 because of the rise in the healthcare expenditure in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The global bovine ephemeral fever vaccines market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for global bovine ephemeral fever vaccines market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the global bovine ephemeral fever vaccines market. The data is available for historic period 2015-2020.
Competitive Landscape and Bovine Ephemeral Fever Vaccines Market Share Analysis
The bovine ephemeral fever vaccines market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to the global bovine ephemeral fever vaccines market.
Some of the major players operating in the bovine ephemeral fever vaccines market are:
- Zoetis Services LLC (U.S.)
- Nutri Pharmax Sdn Bhd (Malaysia)
- Octavoscene (Pty) Ltd (South Africa)
- Kyoto Biken Laboratories, Inc. (Japan)
- Indian Immunologicals Ltd. (India)
- CAVAC Network (France)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES DATA VOLUME
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.1.1 PATENT LANDSCAPE
6.1.2 USPTO NUMBER
6.1.3 PATENT EXPIRY
6.1.4 EPIO NUMBER
6.1.5 PATENT STRENGTH AND QUALITY
6.1.6 PATENT CLAIMS
6.1.7 PATENT CITATIONS
6.1.8 PATENT LITIGATION AND LICENSING
6.1.9 FILE OF PATENT
6.1.10 PATENT RECEIVED CONTRIES
6.1.11 TECHNOLOGY BACKGROUND
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH SPECIALIST
6.8 OTHER KOL SNAPSHOTS
7 EPIDEMIOLOGY
7.1 INCIDENCE OF ALL BY GENDER
7.2 TREATMENT RATE
7.3 MORTALITY RATE
7.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
7.5 PATIENT TREATMENT SUCCESS RATES
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 REGULATORY FRAMEWORK
9.1 REGULATORY APPROVAL PROCESS
9.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
9.3 REGULATORY APPROVAL PATHWAYS
9.4 LICENSING AND REGISTRATION
9.5 POST-MARKETING SURVEILLANCE
9.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
10 GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET, BY VACCINE TYPE
10.1 OVERVIEW
10.2 INACTIVATED VACCINES
10.3 LIVE ATTENUATED VACCINES
10.4 OTHERS
11 GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET, BY ANIMAL TYPE
11.1 OVERVIEW
11.2 CATTLE
11.2.1 HOLSTEIN
11.2.1.1. INACTIVATED VACCINES
11.2.1.2. LIVE ATTENUATED VACCINES
11.2.1.3. OTHERS
11.2.2 ANGUS
11.2.2.1. INACTIVATED VACCINES
11.2.2.2. LIVE ATTENUATED VACCINES
11.2.2.3. OTHERS
11.2.3 HEREFORD
11.2.3.1. INACTIVATED VACCINES
11.2.3.2. LIVE ATTENUATED VACCINES
11.2.3.3. OTHERS
11.2.4 BRAHMAN
11.2.4.1. INACTIVATED VACCINES
11.2.4.2. LIVE ATTENUATED VACCINES
11.2.4.3. OTHERS
11.2.5 OTHERS
11.3 WATER BUFFALO
11.3.1 INACTIVATED VACCINES
11.3.2 LIVE ATTENUATED VACCINES
11.3.3 OTHERS
11.4 OTHERS
12 GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET, BY PACKAGING
12.1 OVERVIEW
12.2 5-DOSES
12.3 10-DOSES
12.4 20-DOSES
12.5 50-DOSES
12.6 OTHERS
13 GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET, BY END USER
13.1 OVERVIEW
13.2 VETERINARY HOSPITALS
13.3 ANIMAL FARMA AND HUSBANDRY
13.4 VETERINARY CLINICS
13.5 LABORATORIES
13.6 OTHERS
14 GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 DIRECT TENDER
14.3 RETAIL SALES
14.3.1 DRUGS STORES
14.3.2 HOSPITAL AND CLINICS BASED PHARMACIES
14.3.3 ONLINE PHARMACY
14.3.4 OTHERS
14.4 OTHERS
15 GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: GLOBAL
15.2 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15.3 COMPANY SHARE ANALYSIS: EUROPE
15.4 COMPANY SHARE ANALYSIS: NORTH AMERICA
15.5 MERGERS & ACQUISITIONS
15.6 NEW PRODUCT DEVELOPMENT & APPROVALS
15.7 EXPANSIONS
15.8 REGULATORY CHANGES
15.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
16 GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET, BY GEOGRAPHY
GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
16.1 NORTH AMERICA
16.1.1 U.S.
16.1.2 CANADA
16.1.3 MEXICO
16.2 EUROPE
16.2.1 GERMANY
16.2.2 FRANCE
16.2.3 U.K.
16.2.4 ITALY
16.2.5 SPAIN
16.2.6 RUSSIA
16.2.7 TURKEY
16.2.8 BELGIUM
16.2.9 REST OF EUROPE
16.3 ASIA-PACIFIC
16.3.1 JAPAN
16.3.2 INDIA
16.3.3 AUSTRALIA
16.3.4 THAILAND
16.3.5 REST OF ASIA-PACIFIC
16.4 MIDDLE EAST
16.4.1 SAUDI ARABIA
16.4.2 UAE
16.4.3 ISRAEL
16.4.4 SOUTH AFRICA
16.4.5 TURKEY
16.4.6 REST OF MIDDLE EAST
16.5 SOUTH AMERICA
16.5.1 PERU
16.5.2 COLOMBIA
16.5.3 OTHERS
16.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
17 GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET, SWOT AND DBMR ANALYSIS
18 GLOBAL BOVINE EPHEMERAL FEVER VACCINES MARKET, COMPANY PROFILE
18.1 GREEN CROSS VETERINARY PRODUCTS CO., LTD.
18.1.1 COMPANY OVERVIEW
18.1.2 REVENUE ANALYSIS
18.1.3 GEOGRAPHIC PRESENCE
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENTS
18.2 ZOETIS INC.
18.2.1 COMPANY OVERVIEW
18.2.2 REVENUE ANALYSIS
18.2.3 GEOGRAPHIC PRESENCE
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 OBP VACCINES
18.3.1 COMPANY OVERVIEW
18.3.2 REVENUE ANALYSIS
18.3.3 GEOGRAPHIC PRESENCE
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENTS
18.4 KYOTO BIKEN LABORATORIES, INC
18.4.1 COMPANY OVERVIEW
18.4.2 REVENUE ANALYSIS
18.4.3 GEOGRAPHIC PRESENCE
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENTS
18.5 KBNP
18.5.1 COMPANY OVERVIEW
18.5.2 REVENUE ANALYSIS
18.5.3 GEOGRAPHIC PRESENCE
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
19 RELATED REPORTS
20 CONCLUSION
21 QUESTIONNAIRE
22 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.