Global Canagliflozin Market
Market Size in USD Billion
CAGR :
% 
 USD
2.75 Billion
USD
4.35 Billion
2024
2032
USD
2.75 Billion
USD
4.35 Billion
2024
2032
| 2025 –2032 | |
| USD 2.75 Billion | |
| USD 4.35 Billion | |
|
|
|
|
Canagliflozin Market Analysis
The global canagliflozin market is primarily driven by the increasing prevalence of type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD). According to the International Diabetes Federation, approximately 537 million adults worldwide were living with diabetes in 2021, and this number is expected to rise to 783 million by 2045. Canagliflozin, as an SGLT2 inhibitor, plays a significant role in managing T2DM by improving glycemic control, and it is also approved for reducing the risk of cardiovascular events in diabetic patients. Moreover, the growing burden of CKD, which affects an estimated 700 million people globally, further strengthens the demand for Canagliflozin, especially as it has demonstrated efficacy in slowing the progression of kidney disease. These factors, along with rising awareness about the drug's dual benefit in diabetes and renal care, are contributing to its widespread adoption across various healthcare settings.
Canagliflozin Market Size
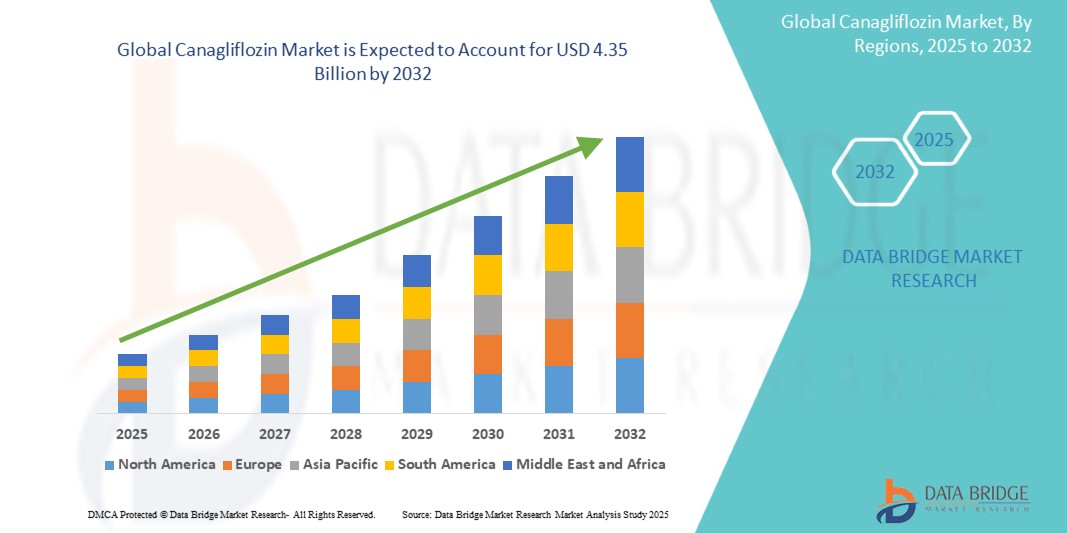
Global canagliflozin market size was valued at USD 2.75 billion in 2024 and is projected to reach USD 4.35 billion by 2032, with a CAGR of 6.10% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Canagliflozin Market Trends
“Shift Towards Generic Versions”
As the patents for branded Canagliflozin expire, the emergence of generic versions is reshaping the market. Generic versions offer a more affordable alternative to the original branded drug, making it accessible to a broader patient base, especially in emerging markets where affordability is a critical factor. This trend reflects a broader move toward cost-effective treatment options in both developed and developing regions. The availability of generics allows healthcare systems to manage growing patient populations with diabetes and chronic kidney disease more sustainably, while also fostering competition and increasing the volume of sales in the market.
Report Scope and Canagliflozin Market Segmentation
|
Attributes |
Canagliflozin Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America |
|
Key Market Players |
Johnson & Johnson Services, Inc. (U.S.), Boehringer Ingelheim International GmbH (Germany), AstraZeneca PLC (U.K.), Merck & Co., Inc. (U.S.), Novartis AG (Switzerland), Eli Lilly and Company (U.S.), Sanofi S.A. (France), Pfizer Inc. (U.S.), Bristol-Myers Squibb Company (U.S.), AbbVie Inc. (U.S.), Gilead Sciences, Inc. (U.S.), GSK plc (U.K.), F. Hoffmann-La Roche AG (Switzerland), Viatris Inc. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), Lupin Limited (India), Dr. Reddy's Laboratories Ltd. (India), Sandoz International GmbH (Germany), Hikma Pharmaceuticals PLC (U.K.), and Sun Pharmaceutical Industries Ltd. (India) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Canagliflozin Market Definition
Canagliflozin is an SGLT2 inhibitor that aids in managing type 2 diabetes by preventing glucose reabsorption in the kidneys, leading to increased glucose excretion. It is also approved for use in patients with heart failure and chronic kidney disease, providing protective cardiovascular and renal effects.
Canagliflozin Market Dynamics
Drivers
- Rising Prevalence of Type 2 Diabetes and Chronic Kidney Disease (CKD)
The rising prevalence of type 2 diabetes and chronic kidney disease (CKD) is a significant driver for the Canagliflozin market. Type 2 diabetes, affecting over 500 million people globally, continues to rise due to factors such as aging populations, sedentary lifestyles, and poor dietary habits. Similarly, CKD, which impacts approximately 700 million people worldwide, is often associated with diabetes and hypertension, further increasing the demand for effective treatments. Canagliflozin, an SGLT2 inhibitor, is widely used for managing type 2 diabetes by lowering blood sugar levels. Its ability to also slow the progression of CKD, a common complication of diabetes, has expanded its therapeutic use, making it essential in managing both conditions. This dual benefit has led to increased adoption among healthcare providers and patients, as the need for comprehensive treatment options that address both diabetes and kidney health becomes more critical in light of the rising disease burden globally. For instance, in June 2024, according to an article published by NCBI, the number of people affected by diabetes in India has risen sharply, from 33 million in 2000 to 72 million in 2021, with projections indicating it could reach 125 million by 2045. This growing prevalence of diabetes is expected to drive demand for effective treatments and therapies, creating significant opportunities for the SGLT2 inhibitors market to expand in India.
- Growing Awareness and Adoption of SGLT2 Inhibitors
The growing awareness and adoption of SGLT2 inhibitors, including canagliflozin, is a significant driver for its market growth. Healthcare professionals are increasingly recognizing the efficacy of SGLT2 inhibitors in managing type 2 diabetes, particularly due to their ability to effectively control blood glucose levels. In addition to glycemic control, canagliflozin offers multiple benefits, such as promoting weight loss, reducing blood pressure, and lowering the risk of cardiovascular events in diabetic patients. These additional therapeutic advantages have made canagliflozin a preferred treatment option for managing not only diabetes but also its associated complications, such as hypertension and cardiovascular disease. As more clinical evidence supports its broad range of benefits, both doctors and patients are more inclined to adopt SGLT2 inhibitors as part of comprehensive diabetes management plans, further boosting their market acceptance and use. This growing preference is fueling the demand for canagliflozin globally.
Opportunities
- Combination Therapies for Broader Indications
The potential for canagliflozin to be used in combination therapies presents a significant opportunity in the global market. As research continues to show its effectiveness in managing not only type 2 diabetes but also cardiovascular disease and chronic kidney disease (CKD), there is a growing interest in combining canagliflozin with other medications to address multiple health concerns. Developing fixed-dose combination products, which pair canagliflozin with other diabetic treatments or medications for heart failure and CKD, could streamline patient regimens, making treatment more convenient and improving adherence. Furthermore, exploring canagliflozin’s use in broader indications, such as heart failure, could expand its therapeutic footprint, offering new options for patients with co-existing conditions. This approach enhances the drug’s value proposition by addressing multiple aspects of chronic diseases in a single treatment, which could drive adoption and market growth, especially in regions with high burdens of diabetes, cardiovascular conditions, and CKD. For instance, in June 2024, according to an article published by Business Standard Private Ltd., Indoco Remedies received USFDA tentative approval for its ANDA for canagliflozin and Metformin Hydrochloride Tablets, a generic version of Invokamet in multiple dosages. This approval presents an opportunity for the company to expand its presence in the growing diabetes treatment market. By offering a combination therapy, it aligns with the trend of broadening treatment options for patients with diabetes and related complications, enhancing its market potential.
- Development of Biosimilars and Generics
As the patents for canagliflozin expire, the development and introduction of generic and biosimilar versions present a significant opportunity to enhance the drug's accessibility and affordability, particularly in low- and middle-income countries. Generic versions of canagliflozin would allow more patients, especially those in economically disadvantaged regions, to access this essential medication at a lower cost. The high price of branded drugs often limits patient access in these areas, but generics can reduce these barriers, leading to wider usage. As generic and biosimilar versions enter the market, they also help stimulate competition, which can further drive down prices and increased availability. This expanded accessibility is crucial in managing the growing global prevalence of type 2 diabetes and chronic kidney disease, where cost-effective treatment options are urgently needed. By making Canagliflozin more affordable, its market penetration can increase, reaching a broader patient population and supporting overall healthcare outcomes. For instance, in November 2023, according to an article published by Lupin, Lupin has received tentative approval from the U.S. FDA for its generic version of Invokamet XR (Canagliflozin and Metformin Hydrochloride Extended-Release Tablets) in various dosages. This approval presents an opportunity for Lupin to expand its presence in the U.S. diabetes market by offering an affordable alternative to the branded product, aligning with the growing trend of developing generics to improve accessibility and address cost barriers.
Restraints/Challenges
- High Cost of Canagliflozin
The high cost of branded canagliflozin continues to be a major restraint in the global market, despite the availability of generics. The initial price of the branded drug can be a significant barrier, particularly in low- and middle-income countries, where patients may face financial challenges in affording the medication. In these regions, healthcare systems often have limited budgets, and out-of-pocket costs for patients can be prohibitive. Even though the introduction of generics over time helps to lower costs and increase accessibility, the premium price of the branded version still restricts the drug’s reach. This pricing issue also impacts countries with limited reimbursement coverage or those without adequate insurance systems, further hindering widespread adoption. As a result, the high cost of canagliflozin limits its market penetration in these areas, affecting its potential to reach a larger patient population, especially in regions where diabetes and chronic kidney disease are prevalent.
- Regulatory Hurdles and Approval Processes
Navigating regulatory hurdles and approval processes presents a significant challenge for the global canagliflozin market. Different regions have their own regulatory frameworks, which can cause delays in the drug’s market entry, particularly when new formulations or expanded indications are involved. For instance, the approval of canagliflozin for additional uses, such as heart failure or chronic kidney disease (CKD), requires comprehensive clinical trials and the submission of extensive data to regulatory bodies. These processes can be both time-consuming and costly, often delaying the drug’s availability in various regions. In emerging markets, where regulatory procedures may be more stringent or slower, these delays are particularly pronounced. This can hinder the adoption of canagliflozin, preventing it from reaching a broader patient population. Ultimately, these regulatory challenges slow down the drug’s market expansion and limit its ability to capitalize on new therapeutic indications.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Canagliflozin Market Scope
The market is segmented on the basis of indication, patient type, therapeutic class, end user, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Indication
- Type 2 Diabetes Mellitus (T2DM)
- Chronic Kidney Disease (CKD)
- Cardiovascular Risk Reduction (CVD)
Patient Type
- Pediatric
- Adult
- Geriatric
Therapeutic Class
- Antidiabetic Drugs
- Renal Care Drugs
- Cardiovascular Drugs
End User
- Hospitals
- Clinics
- Homecare Settings
- Pharmacies/Drugstores
Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
- Online Pharmacies
Canagliflozin Market Regional Analysis
The market is analyzed and market size insights and trends are provided by country, indication, patient type, therapeutic class, end user, and distribution channel as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America is expected to dominate the market due to the high prevalence of type 2 diabetes, supported by advanced healthcare infrastructure that ensures timely diagnosis and treatment. Additionally, strong reimbursement policies and the widespread adoption of SGLT2 inhibitors, including canagliflozin, drive market growth by improving patient access to innovative diabetes therapies.
Asia-Pacific is expected to be the fastest growing due to the rising prevalence of type 2 diabetes, particularly in densely populated countries such as China and India. The increasing access to healthcare services and a growing demand for effective diabetes treatments in emerging economies further fuel market expansion, supported by rising awareness and improving economic conditions.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Canagliflozin Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Canagliflozin Market Leaders Operating in the Market Are:
- Johnson & Johnson Services, Inc.(U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- AstraZeneca PLC (U.K.)
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
- Eli Lilly and Company (U.S.)
- Sanofi S.A. (France)
- Pfizer Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- AbbVie Inc. (U.S.)
- Gilead Sciences, Inc. (U.S.)
- GSK plc (U.K.)
- F. Hoffmann-La Roche AG (Switzerland)
- Viatris Inc. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Lupin Limited (India)
- Dr. Reddy's Laboratories Ltd. (India)
- Sandoz International GmbH (Germany)
- Hikma Pharmaceuticals PLC (U.K.)
- Sun Pharmaceutical Industries Ltd. (India)
Latest Developments in Canagliflozin Market
- In June 2024, Indoco Remedies received tentative approval from the USFDA for its Abbreviated New Drug Application (ANDA) for canagliflozin and Metformin Hydrochloride Tablets, a generic version of Invokamet in various dosages. This approval will enable the company to expand its product portfolio and tap into the growing demand for affordable diabetes treatments in the U.S. market
- In November 2023, Lupin Limited has received tentative approval from the U.S. FDA for its Abbreviated New Drug Application for canagliflozin and Metformin Hydrochloride Extended-Release Tablets, a generic version of Janssen’s Invokamet XR Tablets in various dosages. This approval will enable Lupin to enter the competitive U.S. market for diabetes treatments, boosting its revenue from generics and expanding its product portfolio
- In July 2023, Menarini has become the exclusive distributor of INVOKANA (canagliflozin) and VOKANAMET (canagliflozin and metformin) in the UK and Northern Ireland, following a distribution agreement with Janssen, effective January 2, 2023, with physical distribution beginning July 1, 2023. This partnership allows Menarini to strengthen its market position in the UK and expand its portfolio of diabetes treatments, increasing revenue opportunities
- In February 2023, Zydus Lifesciences Limited has received tentative USFDA approval for its generic canagliflozin and Metformin Hydrochloride Tablets (50 mg/500 mg, 50 mg/1,000 mg, 150 mg/500 mg, and 150 mg/1,000 mg), equivalent to Janssen's Invokamet Tablets. This approval will enable Zydus to expand its presence in the U.S. diabetes market, offering a cost-effective alternative to the branded product
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.