Global Cardasil Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
3.02 Billion
USD
4.30 Billion
2024
2032
USD
3.02 Billion
USD
4.30 Billion
2024
2032
| 2025 –2032 | |
| USD 3.02 Billion | |
| USD 4.30 Billion | |
|
|
|
|
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Size
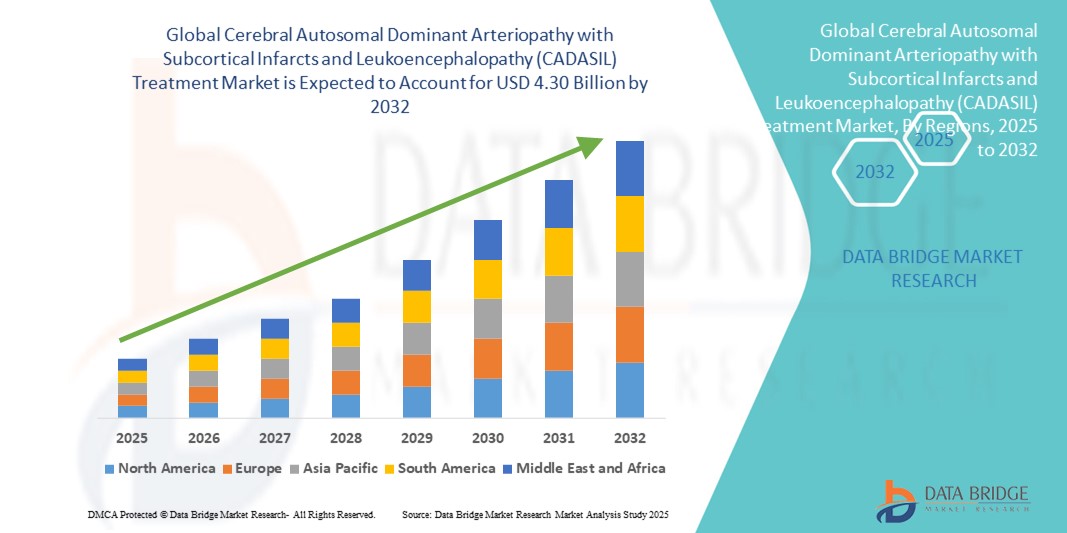
- The global cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market size was valued at USD 3.02 billion in 2024 and is expected to reach USD 4.30 billion by 2032, at a CAGR of 4.50% during the forecast period
- The market growth is largely driven by increasing awareness and improved diagnosis of rare genetic disorders, along with advancements in targeted therapies and personalized medicine for neurological conditions
- Furthermore, rising patient demand for effective, safe, and accessible treatment options for CADASIL is encouraging the development and adoption of innovative therapeutic approaches. These factors are collectively fostering market expansion, thereby significantly enhancing the growth trajectory of the CADASIL treatment industry
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Analysis
- CADASIL treatments, including physical therapy, occupational therapy, thrombolytic therapy, antiplatelet agents, acetylcholinesterase inhibitors, and anticonvulsants, are increasingly vital for managing this rare genetic disorder, helping improve patient outcomes and slow disease progression
- The escalating demand for CADASIL treatments is primarily fueled by advancements in genetic testing and early diagnosis, increasing awareness among healthcare professionals and patients, and growing adoption of precision medicine strategies tailored to individual genetic profiles
- North America dominated the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market with the largest revenue share of 40.5% in 2024, driven by advanced healthcare infrastructure, widespread availability of specialized neurological care, and active research and development by key pharmaceutical and biotech companies, with the U.S. leading in clinical trials and adoption of innovative therapies
- Asia-Pacific is expected to be the fastest growing region in the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market during the forecast period due to rising awareness of rare neurological disorders, expanding healthcare infrastructure, and improving access to advanced diagnostics and therapies in countries such as China and India
- Antiplatelet Agents dominated the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market in 2024 with a market share of 40.6%, reflecting their widespread use in preventing stroke and managing vascular complications associated with the disease
Report Scope and Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Segmentation
|
Attributes |
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Trends
Advancements in Genetic Testing and Targeted Therapies
- A significant trend in the global CADASIL treatment market is the increasing integration of advanced genetic testing with the development of targeted therapeutic options. These innovations are enhancing early diagnosis and enabling more personalized treatment strategies for patients
- For instance, next-generation sequencing panels allow clinicians to identify NOTCH3 gene mutations with high accuracy, facilitating early intervention and improved patient management. Similarly, emerging therapies are being tailored to address vascular dysfunction and neurological symptoms specific to CADASIL patients
- Advances in treatment approaches, such as personalized medication regimens and neuroprotective strategies, enable better management of stroke-such as episodes, cognitive decline, and migraine symptoms. Ongoing clinical trials are also exploring disease-modifying therapies aimed at slowing disease progression
- The integration of these diagnostic and therapeutic innovations with electronic health records and remote monitoring systems supports more coordinated care, allowing clinicians to track patient responses and optimize treatment plans over time
- This trend toward precision medicine and early intervention is fundamentally reshaping patient expectations for CADASIL management. Consequently, pharmaceutical and biotech companies are investing in research for targeted drugs and supportive therapies that cater specifically to the disease’s genetic and clinical profile
- The adoption of advanced diagnostics and personalized therapies is rapidly growing across both hospitals and specialty clinics, as patients and healthcare providers prioritize timely, effective, and tailored treatment solutions
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Dynamics
Driver
Rising Awareness and Early Diagnosis Driving Treatment Adoption
- The Increasing awareness of CADASIL among healthcare professionals and patients, combined with advancements in genetic testing, is a key driver for the growing demand for treatment options
- For instance, in 2024, several clinical centers in North America and Europe expanded CADASIL screening programs using advanced genetic panels to identify patients at risk earlier. These initiatives are expected to drive the market growth in the forecast period
- Early diagnosis enables timely interventions with antiplatelet agents, supportive therapies, and symptom management, improving patient outcomes and reducing the risk of severe complications
- In addition, the growing emphasis on precision medicine and personalized treatment plans encourages healthcare providers to adopt novel therapeutic approaches
- Increasing patient awareness and advocacy for rare neurological disorders are prompting hospitals and specialty clinics to offer comprehensive care, including genetic counseling, treatment monitoring, and supportive therapies
Restraint/Challenge
High Treatment Costs and Limited Awareness in Emerging Regions
- The high cost of advanced diagnostic testing and targeted therapies for CADASIL presents a significant barrier to broader market penetration, particularly in developing regions with limited healthcare infrastructure
- For instance, the price of genetic testing and novel therapeutic options may be prohibitive for some patients, limiting accessibility and adoption
- In addition, lack of awareness and understanding of CADASIL among general practitioners and patients in emerging markets delays diagnosis and treatment initiation
- Addressing these challenges through patient education, healthcare provider training, insurance coverage expansion, and development of cost-effective therapies will be critical to enhance treatment accessibility
- Continued efforts by pharmaceutical companies and healthcare organizations to provide affordable solutions, coupled with awareness campaigns, will be vital for sustained market growth and improved patient outcomes
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Scope
The market is segmented on the basis of treatment type, drugs, route of administration, end-users, and distribution channel.
- By Treatment Type
On the basis of treatment type, the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market is segmented into physical therapy, occupational therapy, thrombolytic therapy, and others. The Physical Therapy segment dominated the market with the largest revenue share of around 42.5% in 2024. Physical therapy is widely used to manage mobility impairments, stroke-such as episodes, and overall neurological deterioration associated with CADASIL. It helps patients maintain functional independence, improves quality of life, and reduces the long-term burden on caregivers. The segment’s dominance is supported by strong adoption in hospitals and specialty clinics, where structured rehabilitation programs are implemented. In addition, the availability of trained therapists and established protocols for rare neurological disorders drives the continuous demand for physical therapy in CADASIL management.
The Occupational Therapy segment is expected to witness the fastest growth rate from 2025 to 2032, owing to increasing recognition of its role in helping patients maintain daily living activities despite cognitive or motor impairments. Occupational therapy focuses on enabling patients to adapt to functional challenges, using assistive devices and personalized interventions to preserve autonomy. Growing awareness among healthcare providers and caregivers about the benefits of early occupational therapy intervention, combined with expanding specialty clinic services in developed and emerging markets, is driving the adoption of this treatment.
- By Drugs
On the basis of drugs, the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market is segmented into Antiplatelet Agents, Acetylcholinesterase Inhibitors, Anticonvulsants, and Others. The Antiplatelet Agents segment dominated the market with 40.6% share in 2024, as these drugs are widely prescribed to prevent stroke and vascular complications, which are the primary risks in CADASIL patients. Their effectiveness in reducing recurrent ischemic events makes them a standard treatment option across hospitals and specialty clinics. Market dominance is further supported by high patient adherence to oral antiplatelet therapy, relatively lower cost compared to emerging targeted therapies, and strong clinician familiarity with these drugs.
The Acetylcholinesterase Inhibitors segment is anticipated to be the fastest growing during forecast period, driven by ongoing clinical research exploring their potential to improve cognitive functions and mitigate dementia-related symptoms in CADASIL patients. Increasing focus on symptomatic management of cognitive impairment and the growing number of patients diagnosed early due to advanced genetic testing are accelerating adoption. Pharmaceutical companies are also investing in developing newer formulations with improved efficacy and tolerability, contributing to the rapid growth of this segment.
- By Route of Administration
On the basis of route of administration, the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market is segmented into Oral and Parenteral. The Oral segment dominated the market in 2024 with 65% share, reflecting its convenience, ease of administration, and strong patient compliance. Most antiplatelet agents, acetylcholinesterase inhibitors, and anticonvulsants are available in oral form, allowing patients to manage therapy at home with minimal clinical supervision. Hospitals and homecare providers prefer oral medications for outpatient management due to lower costs and easier long-term adherence.
The Parenteral segment is expected to witness the fastest growth during the forecast period due to the development of injectable therapies for disease-modifying interventions and targeted neuroprotective agents. As new biologics and advanced therapeutics enter clinical trials, parenteral administration becomes necessary to ensure bioavailability and efficacy, particularly in patients with severe or progressing symptoms. Expansion of specialty clinic infrastructure and training for parenteral therapy administration is also contributing to this growth
- By End-Users
On the basis of end-users, the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market is segmented into Hospitals, Homecare, Specialty Clinics, and Others. The Hospitals segment dominated the market with 50% share in 2024, as they provide structured care, access to specialized neurologists, rehabilitation programs, and continuous monitoring of treatment outcomes. Hospitals serve as the primary point for diagnosis, initiation of therapy, and management of complications, making them critical in CADASIL care. The availability of multidisciplinary teams and integrated treatment approaches in hospitals further strengthens this segment’s market position.
The Specialty Clinics segment is expected to witness the fastest growth during the forecast period, driven by the increasing establishment of rare disease centers and neurology-focused clinics. These clinics offer personalized patient care, access to advanced diagnostic tools, and participation in clinical trials, which appeals to CADASIL patients seeking specialized treatment. Growing awareness of CADASIL and early referral from primary care providers are accelerating the adoption of specialty clinic services, particularly in developed regions.
- By Distribution Channel
On the basis of distribution channel, the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market is segmented into Hospital Pharmacies, Retail Pharmacies, and Others. The Hospital Pharmacies segment dominated the market with 55% share in 2024, due to the structured dispensing of prescribed therapies, especially antiplatelet agents and supportive medications, and the close monitoring of patient adherence. Hospitals often integrate pharmacy services with clinical care, ensuring timely access to medication and reducing the risk of complications.
The Retail Pharmacies segment is expected to witness the fastest growth during forecast period, supported by increasing homecare adoption, improved drug availability in local pharmacies, and rising awareness among patients about CADASIL management. Expanding retail pharmacy networks in emerging economies and the growth of e-pharmacy services also facilitate easier access to medications, driving this segment’s adoption. The increasing popularity of e-pharmacy services also enables easier access to medications, boosting the segment’s adoption.
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Regional Analysis
- North America dominated the cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market with the largest revenue share of 40.5% in 2024, driven by advanced healthcare infrastructure, widespread availability of specialized neurological care, and active research and development by key pharmaceutical and biotech companies, with the U.S. leading in clinical trials and adoption of innovative therapies
- Patients and healthcare providers in the region prioritize early diagnosis, access to multidisciplinary care teams, and advanced treatment options such as antiplatelet therapy and supportive rehabilitation, which are essential for managing CADASIL’s progressive neurological symptoms
- The widespread adoption of genetic testing, coupled with well-established clinical guidelines and strong research and development activity by pharmaceutical and biotech companies, further reinforces North America’s leading position in the CADASIL treatment market
The U.S. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Insight
The U.S. cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market captured the largest revenue share in North America in 2024, driven by the widespread availability of advanced diagnostic tools, including genetic testing for NOTCH3 mutations, and growing awareness of rare neurological disorders. Patients increasingly prioritize early diagnosis and effective management of stroke-such as episodes, cognitive decline, and migraine symptoms associated with CADASIL. The adoption of antiplatelet therapy, rehabilitation programs, and targeted supportive treatments in hospitals and specialty clinics further strengthens market growth. Moreover, robust research and development activity, along with active participation in clinical trials, supports the introduction of innovative therapies, fueling market expansion in the U.S.
Europe Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Insight
The Europe cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market is projected to expand at a substantial CAGR during the forecast period, primarily driven by growing awareness of rare genetic disorders, increasing investment in neurology research, and well-established healthcare infrastructure. Rising urbanization and the expansion of specialized neurology centers contribute to higher diagnosis rates, while government programs supporting rare disease management encourage patients to seek appropriate treatment. Europe’s strong focus on patient safety, early intervention, and integration of clinical care with genetic counseling supports the growing adoption of CADASIL therapies across hospitals and specialty clinics.
U.K. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Insight
The U.K. cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by heightened awareness among healthcare providers and patients regarding early detection and disease management. Increasing prevalence of stroke and cognitive disorders, combined with strong healthcare policies supporting rare disease care, motivates the adoption of advanced diagnostic and treatment solutions. In addition, the presence of specialized neurology clinics, along with established e-health and telemedicine infrastructure, facilitates patient access to treatments and ongoing monitoring, further driving market growth.
Germany Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Insight
The Germany cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of rare neurological disorders and a strong focus on precision medicine. Germany’s well-developed healthcare infrastructure, high accessibility to specialized neurology centers, and emphasis on research and innovation promote the adoption of CADASIL treatments. The integration of genetic testing, preventive care, and personalized therapy options into standard healthcare practice supports patient-centric management and contributes to the steady growth of the market in Germany.
Asia-Pacific Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Insight
The Asia-Pacific cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market is poised to grow at the fastest CAGR during the forecast period, driven by increasing awareness of rare neurological disorders, expanding healthcare infrastructure, and rising availability of advanced diagnostic and treatment options in countries such as China, Japan, and India. Government initiatives promoting rare disease management and improved access to specialty care centers are accelerating early diagnosis and treatment. In addition, the rising number of neurology specialists and growing patient advocacy for rare diseases are encouraging the adoption of CADASIL therapies across hospitals and clinics in the region.
Japan Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Insight
The Japan cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market is gaining momentum due to the country’s advanced healthcare system, high patient awareness, and focus on early diagnosis through genetic testing. Increasing prevalence of stroke-such as episodes and cognitive impairments associated with CADASIL drives the need for timely intervention using antiplatelet therapy and supportive care. Integration of CADASIL management into specialized neurology centers and telemedicine platforms facilitates continuous patient monitoring, improving outcomes and fueling market growth.
India Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Insight
The India cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) treatment market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to increasing awareness of rare neurological disorders, rapid expansion of healthcare infrastructure, and rising availability of advanced diagnostic facilities. Patients are increasingly seeking early diagnosis and treatment through hospitals and specialty clinics, supported by government initiatives for rare disease care. Moreover, growing access to affordable therapies, increasing numbers of neurology specialists, and enhanced patient education are key factors propelling the CADASIL treatment market in India.
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market Share
The Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment industry is primarily led by well-established companies, including:
- Abbvie Inc. (U.S.)
- Fresenius Kabi AG Germany)
- Hikma Pharmaceuticals PLC (U.K.)
- Athenex, Inc (U.S.)
- Eisai Co., Ltd (Japan)
- Jubilant Life Sciences Ltd. (India)
- Dr. Reddy’s Laboratories Ltd (India)
- Zydus Cadila (India)
- Aurobindo Pharma (India)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Novartis AG (Germany)
- WOCKHARDT (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Cipla Inc. (India)
- Unichem Laboratories (India)
- Stemedica Cell Technologies (U.S.)
- Pfizer Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Bayer AG (Germany)
- Takeda Pharmaceutical Company Limited (Japan)
What are the Recent Developments in Global Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) Treatment Market?
- In June 2025, a comprehensive review published on arXiv highlighted emerging immunotherapy and cell therapy approaches for CADASIL treatment. The review discussed various strategies, including gene editing, cell-based therapies, and immune interventions, aiming to modify the disease course and improve long-term outcomes for patients with CADASIL
- In June 2025, cureCADASIL hosted its annual Patient-Investigator Meeting in Itasca, Illinois, focusing on advancing research and fostering collaboration between patients and researchers. The event featured presentations on recent scientific developments and provided a platform for patients to share experiences, contributing to a more patient-centered approach in CADASIL research
- In December 2024, the CERVCO symposium brought together leading researchers and clinicians to discuss advancements in CADASIL research and treatment. The event emphasized the importance of international collaboration and patient-centered care in tackling rare diseases such as CADASIL, fostering a global effort to improve patient outcomes
- In July 2024, a study published in the Journal of Neurology, Neurosurgery & Psychiatry provided updated risk estimates for CADASIL patients based on a 23-year study of 555 individuals. The findings suggest that the clinical phenotype of the disease may be improving over time, possibly due to a reduction in vascular risk factors such as smoking. This research is vital for patient counseling, as it provides a more nuanced understanding of the disease's progression and emphasizes the importance of managing cardiovascular risk factors
- In January 2023, a study published in EMBO Molecular Medicine reported the successful use of an active immunization therapy in a mouse model of CADASIL. Researchers developed a novel approach to specifically target the mutated NOTCH3 protein, which aggregates around vascular smooth muscle cells in CADASIL patients
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 EPIDEMIOLOGY
11.1 INCIDENCE OF ALL BY GENDER
11.2 TREATMENT RATE
11.3 MORTALITY RATE
11.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
11.5 PATIENT TREATMENT SUCCESS RATES
12 REGULATORY COMPLIANCE
12.1 REGULATORY AUTHORITIES
12.2 REGULATORY CLASSIFICATIONS
12.2.1 CLASS I
12.2.2 CLASS II
12.2.3 CLASS III
12.3 REGULATORY SUBMISSIONS
12.4 INTERNATIONAL HARMONIZATION
12.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
12.6 REGULATORY CHALLENGES AND STRATEGIES
13 PIPELINE ANALYSIS
13.1 CLINICAL TRIALS AND PHASE ANALYSIS
13.2 DRUG THERAPY PIPELINE
13.3 PHASE III CANDIDATES
13.4 PHASE II CANDIDATES
13.5 PHASE I CANDIDATES
13.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL)
Company Name Product Name
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE FOR CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL)
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved but Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE FOR CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL)
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE FOR CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL)
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL)
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
14 REIMBURSEMENT FRAMEWORK
15 OPPUTUNITY MAP ANALYSIS
16 VALUE CHAIN ANALYSIS
17 HEALTHCARE ECONOMY
17.1 HEALTHCARE EXPENDITURE
17.2 CAPITAL EXPENDITURE
17.3 CAPEX TRENDS
17.4 CAPEX ALLOCATION
17.5 FUNDING SOURCES
17.6 INDUSTRY BENCHMARKS
17.7 GDP RATION IN OVERALL GDP
17.8 HEALTHCARE SYSTEM STRUCTURE
17.9 GOVERNMENT POLICIES
17.1 ECONOMIC DEVELOPMENT
18 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, BY TREATMENT TYPE
18.1 OVERVIEW
18.2 MEDICATION
18.2.1 ANTIPLATELET AGENTS
18.2.1.1. BY TYPE
18.2.1.1.1. ASPIRIN
18.2.1.1.2. CLOPIDOGREL
18.2.1.2. BY ROUTE OF ADMINISTRATION
18.2.1.2.1. ORAL
18.2.1.2.2. PARENTERAL
18.2.1.2.3. OTHERS
18.2.2 ACETYLCHOLINESTERASE INHIBITOR
18.2.2.1. BY TYPE
18.2.2.1.1. DONEPEZIL
18.2.2.1.2. RIVASTIGMINE
18.2.2.2. BY ROUTE OF ADMINISTRATION
18.2.2.2.1. ORAL
18.2.2.2.2. PARENTERAL
18.2.2.2.3. OTHERS
18.2.3 ANTICONVULSANT
18.2.3.1. BY TYPE
18.2.3.1.1. LEVETIRACETAM
18.2.3.1.2. LAMOTRIGINE
18.2.3.2. BY ROUTE OF ADMINISTRATION
18.2.3.2.1. ORAL
18.2.3.2.2. PARENTERAL
18.2.3.2.3. OTHERS
18.2.4 ANTI-HYPERTENSIVE
18.2.4.1. BY TYPE
18.2.4.1.1. AMLODIPINE
18.2.4.1.2. LOSARTAN
18.2.4.1.3. ATENOLOL
18.2.4.2. BY ROUTE OF ADMINISTRATION
18.2.4.2.1. ORAL
18.2.4.2.2. PARENTERAL
18.2.4.2.3. OTHERS
18.2.5 ANTI-DEPRESSANTS
18.2.5.1. BY ROUTE OF ADMINISTRATION
18.2.5.1.1. ORAL
18.2.5.1.2. PARENTERAL
18.2.5.1.3. OTHERS
18.2.6 EMERGING/PIPELINE DRUGS
18.2.7 OTHERS
18.3 THERAPY
18.3.1 PHYSICAL THERAPY
18.3.1.1. MARKET VALUE (USD MN)
18.3.1.2. MARKET VOLUME (MILLION)
18.3.1.3. ASP (USD)
18.3.1.4. COST OF THERAPY
18.3.2 OCCUPATIONAL THERAPY
18.3.2.1. MARKET VALUE (USD MN)
18.3.2.2. MARKET VOLUME (MILLION)
18.3.2.3. ASP (USD)
18.3.2.4. COST OF THERAPY
18.3.3 SPEECH THERAPY
18.3.3.1. MARKET VALUE (USD MN)
18.3.3.2. MARKET VOLUME (MILLION)
18.3.3.3. ASP (USD)
18.3.3.4. COST OF THERAPY
18.3.4 THROMBOLYTIC THERAPY
18.3.4.1. MARKET VALUE (USD MN)
18.3.4.2. MARKET VOLUME (MILLION)
18.3.4.3. ASP (USD)
18.3.4.4. COST OF THERAPY
18.3.5 VASCULAR NEUROLOGY
18.3.5.1. MARKET VALUE (USD MN)
18.3.5.2. MARKET VOLUME (MILLION)
18.3.5.3. ASP (USD)
18.3.5.4. COST OF THERAPY
18.3.6 DERMATOLOGY
18.3.6.1. MARKET VALUE (USD MN)
18.3.6.2. MARKET VOLUME (MILLION)
18.3.6.3. ASP (USD)
18.3.6.4. COST OF THERAPY
18.3.7 PSYCHIATRY
18.3.7.1. MARKET VALUE (USD MN)
18.3.7.2. MARKET VOLUME (MILLION)
18.3.7.3. ASP (USD)
18.3.7.4. COST OF THERAPY
18.3.8 NEUROPSYCHOLOGY
18.3.8.1. MARKET VALUE (USD MN)
18.3.8.2. MARKET VOLUME (MILLION)
18.3.8.3. ASP (USD)
18.3.8.4. COST OF THERAPY
18.3.9 OTHERS
19 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, BY TYPE
19.1 OVERVIEW
19.2 SYMPTOMATIC TREATMENT
19.3 SUPPORTIVE TREATMENT
20 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, BY DRUGS TYPE
20.1 OVERVIEW
20.2 BRANDED
20.3 GENERIC
21 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
21.1 OVERVIEW
21.2 ORAL
21.2.1 TABLETS
21.2.2 CAPSULES
21.2.3 OTHERS
21.3 PARENTERAL
21.4 OTHERS
22 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, BY AGE GROUP
22.1 OVERVIEW
22.2 PEDIATRIC
22.3 ADULTS
22.4 GERIATRIC
23 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, BY GENDER
23.1 OVERVIEW
23.2 MALE
23.3 FEMALE
24 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, BY END USER
24.1 OVERVIEW
24.2 HOSPITALS
24.2.1 BY TYPE
24.2.1.1. PUBLIC
24.2.1.2. PRIVATE
24.2.2 BY TIER
24.2.2.1. TIER 1
24.2.2.2. TIER 2
24.2.2.3. TIER 3
24.3 SPECIALTY CLINICS
24.4 HOMECARE
24.5 OTHER
25 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, BY DISTRIBUTION CHANNEL
25.1 OVERVIEW
25.2 DIRECT TENDERS
25.3 RETAIL SALES
25.3.1 HOSPITAL PHARMACIES
25.3.2 RETAIL PHARMACIES
25.3.3 OTHERS
25.4 OTHERS
26 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, COMPANY LANDSCAPE
26.1 COMPANY SHARE ANALYSIS: GLOBAL
26.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
26.3 COMPANY SHARE ANALYSIS: EUROPE
26.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
26.5 MERGERS & ACQUISITIONS
26.6 NEW PRODUCT DEVELOPMENT & APPROVALS
26.7 EXPANSIONS
26.8 REGULATORY CHANGES
26.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
27 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, SWOT AND DBMR ANALYSIS
28 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, BY REGION
GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
28.1 NORTH AMERICA
28.1.1 U.S.
28.1.2 CANADA
28.1.3 MEXICO
28.2 EUROPE
28.2.1 GERMANY
28.2.2 FRANCE
28.2.3 U.K.
28.2.4 HUNGARY
28.2.5 LITHUANIA
28.2.6 AUSTRIA
28.2.7 IRELAND
28.2.8 NORWAY
28.2.9 POLAND
28.2.10 ITALY
28.2.11 SPAIN
28.2.12 RUSSIA
28.2.13 TURKEY
28.2.14 NETHERLANDS
28.2.15 SWITZERLAND
28.2.16 REST OF EUROPE
28.3 ASIA-PACIFIC
28.3.1 JAPAN
28.3.2 CHINA
28.3.3 SOUTH KOREA
28.3.4 INDIA
28.3.5 AUSTRALIA
28.3.6 SINGAPORE
28.3.7 THAILAND
28.3.8 MALAYSIA
28.3.9 INDONESIA
28.3.10 PHILIPPINES
28.3.11 VIETNAM
28.3.12 REST OF ASIA-PACIFIC
28.4 SOUTH AMERICA
28.4.1 BRAZIL
28.4.2 ARGENTINA
28.4.3 PERU
28.4.4 COLOMBIA
28.4.5 VENEZUELA
28.4.6 REST OF SOUTH AMERICA
28.5 MIDDLE EAST AND AFRICA
28.5.1 SOUTH AFRICA
28.5.2 SAUDI ARABIA
28.5.3 UAE
28.5.4 EGYPT
28.5.5 KUWAIT
28.5.6 ISRAEL
28.5.7 REST OF MIDDLE EAST AND AFRICA
28.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
29 GLOBAL CEREBRAL AUTOSOMAL DOMINANT ARTERIOPATHY WITH SUBCORTICAL INFARCTS AND LEUKOENCEPHALOPATHY (CADASIL) TREATMENT MARKET, COMPANY PROFILE
29.1 ABBVIE INC
29.1.1 COMPANY OVERVIEW
29.1.2 REVENUE ANALYSIS
29.1.3 GEOGRAPHIC PRESENCE
29.1.4 PRODUCT PORTFOLIO
29.1.5 RECENT DEVELOPMENTS
29.2 NOVARTIS AG.
29.2.1 COMPANY OVERVIEW
29.2.2 REVENUE ANALYSIS
29.2.3 GEOGRAPHIC PRESENCE
29.2.4 PRODUCT PORTFOLIO
29.2.5 RECENT DEVELOPMENTS
29.3 SANOFI
29.3.1 COMPANY OVERVIEW
29.3.2 REVENUE ANALYSIS
29.3.3 GEOGRAPHIC PRESENCE
29.3.4 PRODUCT PORTFOLIO
29.3.5 RECENT DEVELOPMENTS
29.4 PFIZER INC.
29.4.1 COMPANY OVERVIEW
29.4.2 REVENUE ANALYSIS
29.4.3 GEOGRAPHIC PRESENCE
29.4.4 PRODUCT PORTFOLIO
29.4.5 RECENT DEVELOPMENTS
29.5 F. HOFFMANN-LA ROCHE LTD.
29.5.1 COMPANY OVERVIEW
29.5.2 REVENUE ANALYSIS
29.5.3 GEOGRAPHIC PRESENCE
29.5.4 PRODUCT PORTFOLIO
29.5.5 RECENT DEVELOPMENTS
29.6 EUROAPI
29.6.1 COMPANY OVERVIEW
29.6.2 REVENUE ANALYSIS
29.6.3 GEOGRAPHIC PRESENCE
29.6.4 PRODUCT PORTFOLIO
29.6.5 RECENT DEVELOPMENTS
29.7 CIPLA.
29.7.1 COMPANY OVERVIEW
29.7.2 REVENUE ANALYSIS
29.7.3 GEOGRAPHIC PRESENCE
29.7.4 PRODUCT PORTFOLIO
29.7.5 RECENT DEVELOPMENTS
29.8 AAMORB PHARMACEUTICALS PRIVATE LIMITED.
29.8.1 COMPANY OVERVIEW
29.8.2 REVENUE ANALYSIS
29.8.3 GEOGRAPHIC PRESENCE
29.8.4 PRODUCT PORTFOLIO
29.8.5 RECENT DEVELOPMENTS
29.9 AS PHARMA.
29.9.1 COMPANY OVERVIEW
29.9.2 REVENUE ANALYSIS
29.9.3 GEOGRAPHIC PRESENCE
29.9.4 PRODUCT PORTFOLIO
29.9.5 RECENT DEVELOPMENTS
29.1 AKESISS
29.10.1 COMPANY OVERVIEW
29.10.2 REVENUE ANALYSIS
29.10.3 GEOGRAPHIC PRESENCE
29.10.4 PRODUCT PORTFOLIO
29.10.5 RECENT DEVELOPMENTS
29.11 ALKEM
29.11.1 COMPANY OVERVIEW
29.11.2 REVENUE ANALYSIS
29.11.3 GEOGRAPHIC PRESENCE
29.11.4 PRODUCT PORTFOLIO
29.11.5 RECENT DEVELOPMENTS
29.12 ARISTO
29.12.1 COMPANY OVERVIEW
29.12.2 REVENUE ANALYSIS
29.12.3 GEOGRAPHIC PRESENCE
29.12.4 PRODUCT PORTFOLIO
29.12.5 RECENT DEVELOPMENTS
29.13 BIOCON
29.13.1 COMPANY OVERVIEW
29.13.2 REVENUE ANALYSIS
29.13.3 GEOGRAPHIC PRESENCE
29.13.4 PRODUCT PORTFOLIO
29.13.5 RECENT DEVELOPMENTS
29.14 DR. REDDY’S LABORATORIES LTD.
29.14.1 COMPANY OVERVIEW
29.14.2 REVENUE ANALYSIS
29.14.3 GEOGRAPHIC PRESENCE
29.14.4 PRODUCT PORTFOLIO
29.14.5 RECENT DEVELOPMENTS
29.15 BAYER AG
29.15.1 COMPANY OVERVIEW
29.15.2 REVENUE ANALYSIS
29.15.3 GEOGRAPHIC PRESENCE
29.15.4 PRODUCT PORTFOLIO
29.15.5 RECENT DEVELOPMENTS
29.16 PIRAMAL ENTERPRISES LTD.
29.16.1 COMPANY OVERVIEW
29.16.2 REVENUE ANALYSIS
29.16.3 GEOGRAPHIC PRESENCE
29.16.4 PRODUCT PORTFOLIO
29.16.5 RECENT DEVELOPMENTS
29.17 ZYDUS GROUP.
29.17.1 COMPANY OVERVIEW
29.17.2 REVENUE ANALYSIS
29.17.3 GEOGRAPHIC PRESENCE
29.17.4 PRODUCT PORTFOLIO
29.17.5 RECENT DEVELOPMENTS
29.18 TEVA PHARMACEUTICAL INDUSTRIES LTD.
29.18.1 COMPANY OVERVIEW
29.18.2 REVENUE ANALYSIS
29.18.3 GEOGRAPHIC PRESENCE
29.18.4 PRODUCT PORTFOLIO
29.18.5 RECENT DEVELOPMENTS
29.19 TORRENT PHARMACEUTICALS LTD
29.19.1 COMPANY OVERVIEW
29.19.2 REVENUE ANALYSIS
29.19.3 GEOGRAPHIC PRESENCE
29.19.4 PRODUCT PORTFOLIO
29.19.5 RECENT DEVELOPMENTS
29.2 GLENMARK PHARMACEUTICALS LTD.
29.20.1 COMPANY OVERVIEW
29.20.2 REVENUE ANALYSIS
29.20.3 GEOGRAPHIC PRESENCE
29.20.4 PRODUCT PORTFOLIO
29.20.5 RECENT DEVELOPMENTS
29.21 AMNEAL PHARMACEUTICALS LLC.
29.21.1 COMPANY OVERVIEW
29.21.2 REVENUE ANALYSIS
29.21.3 GEOGRAPHIC PRESENCE
29.21.4 PRODUCT PORTFOLIO
29.21.5 RECENT DEVELOPMENTS
29.22 BRISTOL-MYERS SQUIBB COMPANY
29.22.1 COMPANY OVERVIEW
29.22.2 REVENUE ANALYSIS
29.22.3 GEOGRAPHIC PRESENCE
29.22.4 PRODUCT PORTFOLIO
29.22.5 RECENT DEVELOPMENTS
29.23 ELI LILLY AND COMPANY.
29.23.1 COMPANY OVERVIEW
29.23.2 REVENUE ANALYSIS
29.23.3 GEOGRAPHIC PRESENCE
29.23.4 PRODUCT PORTFOLIO
29.23.5 RECENT DEVELOPMENTS
29.24 BOEHRINGER INGELHEIM INTERNATIONAL GMBH.
29.24.1 COMPANY OVERVIEW
29.24.2 REVENUE ANALYSIS
29.24.3 GEOGRAPHIC PRESENCE
29.24.4 PRODUCT PORTFOLIO
29.24.5 RECENT DEVELOPMENTS
29.25 LANNETT
29.25.1 COMPANY OVERVIEW
29.25.2 REVENUE ANALYSIS
29.25.3 GEOGRAPHIC PRESENCE
29.25.4 PRODUCT PORTFOLIO
29.25.5 RECENT DEVELOPMENTS
29.26 EISAI CO., LTD.
29.26.1 COMPANY OVERVIEW
29.26.2 REVENUE ANALYSIS
29.26.3 GEOGRAPHIC PRESENCE
29.26.4 PRODUCT PORTFOLIO
29.26.5 RECENT DEVELOPMENTS
29.27 IONIS PHARMACEUTICALS
29.27.1 COMPANY OVERVIEW
29.27.2 REVENUE ANALYSIS
29.27.3 GEOGRAPHIC PRESENCE
29.27.4 PRODUCT PORTFOLIO
29.27.5 RECENT DEVELOPMENTS
29.28 DALTON PHARMA SERVICES
29.28.1 COMPANY OVERVIEW
29.28.2 REVENUE ANALYSIS
29.28.3 GEOGRAPHIC PRESENCE
29.28.4 PRODUCT PORTFOLIO
29.28.5 RECENT DEVELOPMENTS
29.29 HIKMA PHARMACEUTICALS PLC
29.29.1 COMPANY OVERVIEW
29.29.2 REVENUE ANALYSIS
29.29.3 GEOGRAPHIC PRESENCE
29.29.4 PRODUCT PORTFOLIO
29.29.5 RECENT DEVELOPMENTS
29.3 STERLING PHARMA SOLUTIONS
29.30.1 COMPANY OVERVIEW
29.30.2 REVENUE ANALYSIS
29.30.3 GEOGRAPHIC PRESENCE
29.30.4 PRODUCT PORTFOLIO
29.30.5 RECENT DEVELOPMENTS
30 RELATED REPORTS
31 CONCLUSION
32 QUESTIONNAIRE
33 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.