Global Castleman Disease Drug Market
Market Size in USD Million
CAGR :
% 
 USD
231.78 Million
USD
342.44 Million
2024
2032
USD
231.78 Million
USD
342.44 Million
2024
2032
| 2025 –2032 | |
| USD 231.78 Million | |
| USD 342.44 Million | |
|
|
|
|
Castleman Disease Drug Market Size
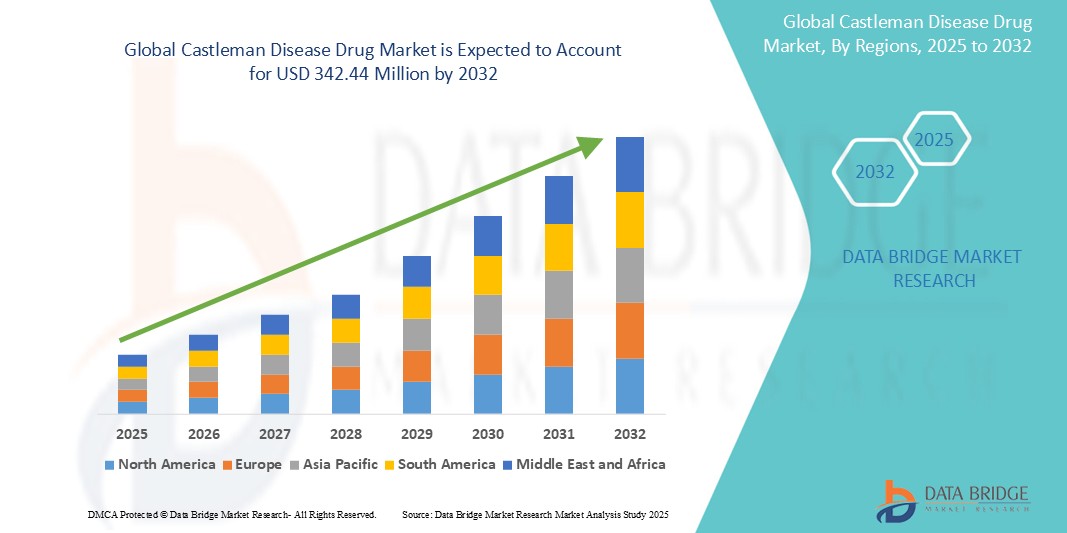
• The global Castleman Disease drug market was valued at USD 231.78 million in 2024 and is expected to reach USD 342.44 million by 2032 and is projected to grow at a CAGR of 5.0%, primarily driven by the increasing global prevalence of Castleman Disease and the rising demand for monoclonal antibodies and immunomodulatory therapies
• This growth is further propelled by factors such as advances in rare disease diagnostics, increased clinical trial activities, and expanding access to targeted biologic treatments in emerging healthcare markets
Castleman Disease Drug Market Analysis
- The increasing need for effective treatment options to manage unicentric and multicentric Castleman Disease, including IL-6 inhibitors and antiviral-based therapies, is boosting demand for immunosuppressive agents, monoclonal antibodies, and supportive therapies
- The market benefits from ongoing R&D into novel biologics, enhanced disease awareness programs, and increased access to early diagnostic and molecular testing tools
- Growth in rare disease registries, supportive orphan drug reimbursement policies, and expanded clinical guidelines for Castleman Disease are expected to further drive market performance, particularly in North America, Europe, and Rest of Asia-Pacific
- North America dominates the Castleman Disease market with a largest market share of 36.4% in 2024 driven by its advanced healthcare infrastructure and high adoption of advanced technologies driven by the increasing elderly population
- Asia-Pacific is expected to be the fastest growing region in the Castleman Disease market during the forecast period due to rising awareness of shingles and its complications, and expanding access to antiviral therapies and vaccines across developing economies. Government initiatives aimed at improving adult immunization coverage and healthcare infrastructure are also contributing to regional market growth
- The Monoclonal Antibodies segment dominates the market with a market share of 34.5%, in 2024 owing to its established role in the standard treatment of herpes zoster infections. Drugs such as acyclovir, valacyclovir, and famciclovir are widely prescribed due to their proven efficacy in reducing the duration and severity of symptoms. Despite the growing adoption of shingles vaccines, especially in developed markets, antiviral drugs remain the primary treatment modality in acute cases due to their accessibility, cost-effectiveness, and familiarity among healthcare providers. Their continued use in managing post-herpetic neuralgia further reinforces their leading position in the market.
Report Scope and Global Castleman Disease Drug Market Segmentation
|
Attributes |
Global Castleman Disease Drug Market Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Castleman Disease Drug Market Trends
“Shift Toward Immunotherapy and Cytokine-Targeted Drug Development”
- One prominent trend in the Global Castleman Disease Drug Market is the growing awareness and improved diagnosis of rare lymphoproliferative disorders, particularly Castleman disease..
- There is a rising demand for IL-6 inhibitors and immunomodulatory agents to reduce systemic inflammation and lymphoproliferation.
- For instance, healthcare providers are increasingly adopting advanced biologics such as siltuximab (Sylvant) and off-label use of rituximab, which are showing promising results in managing multicentric Castleman disease by modulating immune system activity and reducing lymph node inflammation. term disease stabilization
- This trend is significantly reshaping the treatment landscape for Castleman disease, promoting earlier intervention, reducing disease progression, and enhancing survival rates—particularly in patients with the more severe multicentric form.
- The Castleman Disease Drug Market is poised for sustained growth, driven by increased clinical research, regulatory support for orphan drugs, and ongoing advancements in immunotherapy and precision medicine. As global healthcare systems continue to prioritize rare disease management, targeted therapies for Castleman disease will remain a critical focus in improving rare disease care outcomes
Market Dynamics
Driver
“Growing Need Due to Rising Prevalence and Awareness of Rare Diseases”
- The increasing recognition and diagnosis of Castleman disease—particularly multicentric Castleman disease (MCD), which is more severe and life-threatening—are significantly contributing to the growing demand for effective pharmacological treatments
- As awareness of rare and orphan diseases improves globally, more patients are being correctly diagnosed, which drives the demand for specialized therapies, including monoclonal antibodies and immunosuppressive drugs
- The growing prevalence of associated conditions, such as HIV and HHV-8 infections, which are often linked with MCD, further elevates the need for targeted treatments
- Ongoing advances in immunotherapy and precision medicine highlight the necessity for disease-specific drug development, improving treatment efficacy and patient outcomes
- With a greater number of patients seeking medical intervention and access to treatment programs, the demand for Castleman disease drugs continues to rise, supporting long-term market growth
For instance,
- In August 2023, according to a study published by the National Organization for Rare Disorders (NORD), increased awareness initiatives and improved physician education have led to higher diagnosis rates of Castleman disease, which had historically been underrecognized due to symptom overlap with other conditions
- In March 2022, an article published in the Orphanet Journal of Rare Diseases reported that global efforts in rare disease surveillance have uncovered increasing cases of idiopathic multicentric Castleman disease (iMCD), necessitating new treatment strategies and boosting demand for biologic therapies
- As a result of rising disease prevalence and global rare disease advocacy, there is a substantial increase in demand for innovative therapies in the Castleman disease drug market
Opportunity
“Breakthroughs in Targeted Therapies and Orphan Drug Designations”
- The development of targeted therapies, including anti-IL-6 monoclonal antibodies and novel immunomodulators, presents significant opportunities in the Castleman disease drug market
- Orphan drug designations and regulatory incentives such as priority review, market exclusivity, and grant funding are motivating pharmaceutical companies to invest in R&D for this rare condition
- Targeted biologics such as siltuximab and investigational agents under clinical trials show promising results in improving patient outcomes, especially in refractory or relapsed cases
For instance,
- In January 2025, a clinical update published in The Lancet Hematology highlighted that IL-6 inhibitors significantly improved survival rates and symptom management in patients with idiopathic multicentric Castleman disease, showcasing their clinical value in targeted therapy
- In October 2023, the U.S. FDA granted Orphan Drug Designation to a novel IL-6 pathway modulator being developed by a U.S.-based biotech firm, increasing investor interest and fast-tracking development timelines
- The regulatory and scientific momentum surrounding novel therapeutics, backed by orphan drug policies, positions the Castleman disease drug market for substantial growth in the coming years
Restraint/Challenge
“High Treatment Costs Limiting Accessibility”
- The high cost of Castleman disease treatments, particularly biologic therapies, poses a major challenge, restricting accessibility for many patients, especially in low- and middle-income countries
- These therapies, which are often essential for managing multicentric Castleman disease, can cost tens of thousands of dollars annually, putting financial strain on healthcare systems and patients alike
- Limited reimbursement support and lack of universal insurance coverage in several regions further exacerbate the affordability gap
For instance,
- In November 2024, an article by Health Affairs reported that high treatment costs associated with orphan drugs such as siltuximab could exceed USD 100,000 annually, discouraging widespread use despite their clinical efficacy
- In May 2023, a review published in Rare Disease Reports highlighted the financial barriers faced by patients in emerging economies, where access to biologics for rare diseases like Castleman is severely limited due to lack of pricing regulations and reimbursement frameworks
- These economic constraints contribute to disparities in treatment access and patient outcomes, ultimately hindering broader market penetration and the uptake of advanced therapies
Castleman Disease Drug Market Scope
The market is segmented on the basis of drug class, therapy class, end-users, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
Drug Class |
|
|
Therapy type |
|
|
End-Users |
|
|
Distribution Channel |
|
In 2024, the monoclonal antibodies segment dominates the market with the largest share in the Drug Class segment
The monoclonal antibodies segment dominates the Global Castleman Disease Drug Market with the largest share of 34.5% in 2024, owing to their proven clinical efficacy, targeted mechanism of action, and increasing approval for treating both unicentric and multicentric Castleman Disease (UCD and MCD). Agents like siltuximab have demonstrated significant benefits in managing disease progression and improving patient outcomes, particularly in idiopathic multicentric Castleman Disease (iMCD). The growing preference for biologics among healthcare providers and the expanding clinical use of monoclonal antibodies across multiple regions support this segment’s leadership position in the market.
The immunomodulators segment is expected to account for the largest share during the forecast period in the drug type segment
In 2024, the immunomodulators segment dominates the market due to their critical role in modulating immune response, especially in patients who are refractory to standard therapies. These drugs offer broader immunological benefits and are increasingly adopted as part of combination regimens. Their ability to suppress inflammatory cytokine activity and manage systemic symptoms of Castleman Disease has led to rising clinical use. Additionally, ongoing research, increased awareness among clinicians, and supportive treatment guidelines are accelerating the growth of this segment during the forecast period
Castleman Disease Drug Market Regional Analysis
“North America is the Dominant Region in the Castleman Disease Drug Market”
- North America dominates the Castleman disease drug market with a largest market share of 36.4% in 2024, driven by advanced healthcare infrastructure, early disease detection capabilities, and the strong presence of key biopharmaceutical companies involved in rare disease research
- The U.S. holds a significant share due to a higher rate of diagnosis, growing awareness about Castleman disease, and availability of targeted therapies, such as monoclonal antibodies used in treating multicentric Castleman disease (MCD)
- The presence of established reimbursement systems and strong investment in orphan drug development further support the regional market
- In addition, the region benefits from ongoing clinical trials, active patient advocacy groups, and robust regulatory support for orphan drug approvals, all of which continue to drive market expansion across North America
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- The Asia-Pacific region is expected to witness the highest growth rate in the Castleman disease drug market, fueled by improving diagnostic capabilities, increasing healthcare spending, and growing awareness of rare diseases
- Countries such as China, India, and Japan are emerging as important markets due to the rising prevalence of lymphoproliferative disorders and increasing focus on rare disease treatment
- Japan, with its supportive regulatory environment for orphan drugs and presence of leading pharmaceutical companies, remains a key market for Castleman disease treatment innovations
- China and India, with their large patient populations and improving healthcare access, are seeing greater investment in the diagnosis and treatment of rare diseases. The region is also attracting interest from global biopharma companies seeking to expand access to biologics and immunotherapy agents, thus contributing to market growth
Castleman Disease Drug Market Share
The competitive landscape provides a comprehensive overview of the leading market players. This includes company profiles, financial performance, R&D activities, product pipelines, international presence, production capabilities, strategic developments, strengths, weaknesses, and contributions to immunotherapy and rare disease treatment.
The Major Market Leaders Operating in the Market Include:
- Bausch + Lomb (Canada)
- Takagi Seiko Co., Ltd. (Japan)
- Möller-Wedel Optical (Germany)
- Seiler Instrument & Manufacturing Co. (U.S.)
- Nidek Co., Ltd. (Japan)
- Takara Sunoptic Technologies (U.S.)
- Mitaka USA, Inc. (U.S.)
- Visionix (France)
- Medtronic (U.S.)
- Merit Medical Systems (U.S.)
- SOMATEX Medical Technologies GmbH (Germany)
- BD (U.S.)
Latest Developments in Global Castleman Disease Drug Market
- In March 2023, Pfizer announced a Phase 3 study evaluating an investigational IL-6 inhibitor for multicentric Castleman disease (MCD)
- In July 2022, Roche partnered with a clinical research organization to accelerate biologics development for lymphoproliferative disorders
- In 2024, South Korea’s health authority approved a new monoclonal antibody therapy indicated for HHV-8 negative Castleman disease cases
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.