Global Charcot Marie Tooth Disease Market
Market Size in USD Million
CAGR :
% 
 USD
706.10 Million
USD
1,083.64 Million
2024
2032
USD
706.10 Million
USD
1,083.64 Million
2024
2032
| 2025 –2032 | |
| USD 706.10 Million | |
| USD 1,083.64 Million | |
|
|
|
|
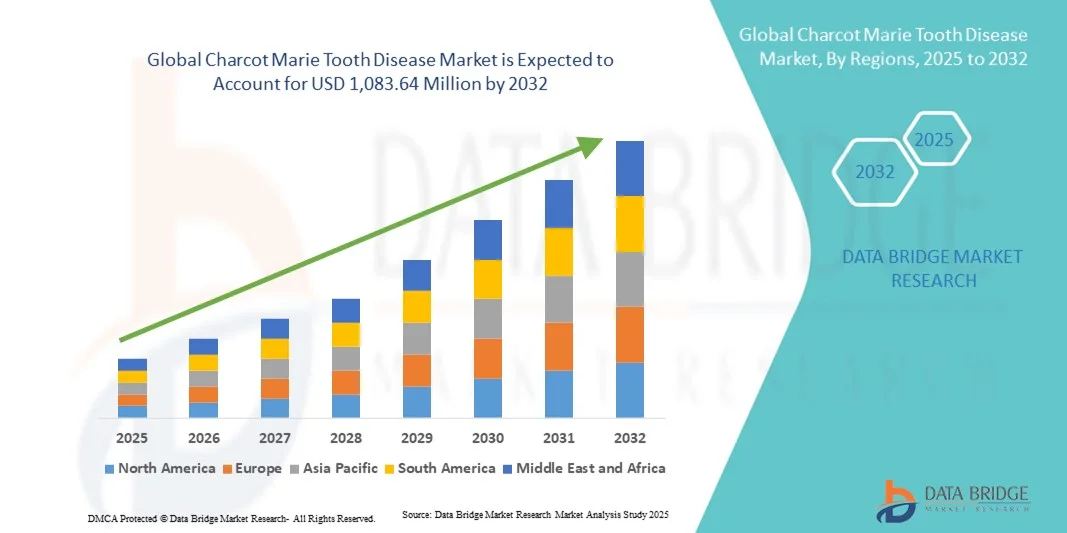
Charcot Marie Tooth Disease Market Size
- The global Charcot Marie Tooth disease market size was valued at USD 706.10 million in 2024 and is expected to reach USD 1,083.64 million by 2032, at a CAGR of 5.50% during the forecast period
- The market's growth is primarily driven by increasing awareness and advancements in genetic testing, which facilitate earlier diagnosis and personalized treatment approaches. In addition, the development of disease-modifying therapies is enhancing treatment options beyond traditional symptomatic care
- Furthermore, the rising prevalence of CMT, estimated to affect approximately 2.6 million individuals globally, is contributing to the growing demand for effective treatments and management strategies
Charcot Marie Tooth Disease Market Analysis
- Charcot-Marie-Tooth (CMT) disease, a group of inherited neurological disorders affecting peripheral nerves, is increasingly recognized as a significant area of focus in neurology and rare disease research
- The market's growth is primarily driven by advancements in genetic testing, which facilitate earlier diagnosis and personalized treatment approaches. In addition, the development of disease-modifying therapies is enhancing treatment options beyond traditional symptomatic care
- North America dominated the CMT disease market with the largest revenue share of 44.42% in 2024. This dominance is attributed to a strong research ecosystem, major companies conducting clinical trials, and early adoption of novel therapies. The high prevalence of CMT in the region further contributes to its market leadership
- Asia-Pacific is expected to be the fastest-growing region in the CMT disease market during the forecast period, with an anticipated CAGR of 6.1%. Factors driving this growth include increasing awareness, improving healthcare infrastructure, and rising disposable incomes in the region
- The CMT1 segment dominated the market with a revenue share of 52.47% in 2024. This dominance is due to the higher prevalence of CMT1 compared to other subtypes, as well as ongoing research and development efforts targeting this form of the disease
Report Scope and Charcot Marie Tooth Disease Market Segmentation
|
Attributes |
Charcot Marie Tooth Disease Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Charcot Marie Tooth Disease Market Trends
Advancements in Genetic Therapies and AI Integration
- A significant and accelerating trend in the global Charcot-Marie-Tooth (CMT) disease market is the growing pipeline of therapeutic candidates, including gene therapies, antisense oligonucleotides (ASOs), RNA-based drugs, and small-molecule modulators. For instance, companies are increasingly focusing on personalized genetic solutions
- For instance, in May 2025, Augustine Therapeutics NV announced the dosing of the first patient in its Phase I clinical trial evaluating AGT-100216, a selective HDAC6 inhibitor for the treatment of CMT
- Similarly, NMD Pharma A/S received FDA orphan drug designation for NMD670, a novel, oral, small molecule inhibitor of the skeletal muscle-specific chloride ion channel ClC-1, targeting CMT
- These advancements in genetic therapies are expected to transform the treatment landscape of CMT, shifting from symptomatic management to curative approaches
- The integration of artificial intelligence (AI) in drug development processes is enhancing the efficiency of identifying potential therapeutic targets and predicting patient responses. For instance, AI-driven platforms are helping accelerate the development of personalized treatments for CMT
- For instance, wearable devices and digital monitoring solutions are increasingly being integrated with CMT clinical studies to track patient mobility and progression, enabling more data-driven treatment plans
- AI-based predictive analytics are being used to identify patients at risk of rapid disease progression. For instance, some biotech startups are using machine learning to anticipate nerve degeneration patterns in CMT patients
Charcot Marie Tooth Disease Market Dynamics
Driver
Increasing Prevalence and Research Advancements
- The increasing prevalence of CMT worldwide is a major factor driving the growth of the global market. For instance, recent epidemiological studies estimate a prevalence of 1 in 2,500 individuals
- For instance, the growing focus on research and development of novel therapeutics, such as gene therapies and small molecules, is expected to fuel market growth
- North America is expected to dominate the global CMT disease market throughout the forecast period, driven by strong research infrastructure and early adoption of innovative therapies. For instance, the Asia Pacific region is projected to grow at the fastest CAGR due to increasing healthcare access, awareness campaigns, and rising adoption of advanced diagnostics
- The growing pipeline of therapeutic candidates and orphan drug designations is one of the most significant drivers accelerating CMT market growth. For instance, multiple clinical trials are underway targeting different genetic subtypes of CMT
- The rising adoption of supportive care technologies, such as customized orthotics and physiotherapy solutions, is boosting overall patient quality of life. For instance, several startups are introducing 3D-printed braces tailored for CMT patients
Restraint/Challenge
Lack of Curative Therapies and Genetic Complexity
- The lack of curative therapies remains a critical restraint hindering the growth of the CMT disease market. For instance, most treatments currently address symptoms only rather than underlying genetic causes
- Despite decades of research, there are no FDA- or EMA-approved disease-modifying treatments. For instance, management primarily relies on physical therapy, orthopedic devices, and pain management
- The genetic heterogeneity of CMT, involving over 100 gene mutations, makes it challenging to design a one-size-fits-all therapy. For instance, this complexity complicates clinical trial design and regulatory approvals
- This therapeutic gap limits patient outcomes and reduces long-term treatment adoption rates. For instance, patients often require personalized care plans, which are not widely available
- Until a truly curative or disease-halting therapy reaches the market, this unmet need will continue to restrain full commercial and clinical potential. For instance, companies are investing heavily in R&D but widespread adoption remains constrained
- Regulatory hurdles and the need for extensive clinical validation are additional challenges. For instance, obtaining orphan drug approvals can be time-consuming, delaying patient access to novel therapies
Charcot Marie Tooth Disease Market Scope
The market is segmented on the basis of type, severity, drug type, treatment type, product type, population, end user, and distribution channel.
- By Type
On the basis of type, the CMT disease market is segmented into CMT1, CMT2, CMT3, and Others. The CMT1 segment dominated the market with the largest market share of 52.47% in 2024, driven by its higher prevalence and well-established clinical awareness. Patients with CMT1 often require long-term management involving medication, physical therapy, and assistive devices. Pharmaceutical companies are focusing R&D efforts on gene therapies and small molecules targeting this subtype. For instance, ADX-71441 is primarily being evaluated for efficacy in CMT1 patients, reflecting the focus on this group. The extensive clinical data and diagnostic infrastructure make CMT1 the dominant contributor to market revenue. Hospitals and specialty clinics prioritize this subtype due to the predictable treatment protocols and patient outcomes.
The CMT2 segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by increasing recognition of its clinical impact and ongoing genetic research. CMT2 patients experience axonal degeneration, requiring innovative interventions and monitoring tools. For instance, AI-assisted genetic testing platforms are improving early detection and personalized treatment planning for CMT2. Rising awareness of this subtype and investment in precision medicine contribute to rapid market growth. Emerging therapies targeting specific mutations are accelerating adoption. Healthcare providers are increasingly integrating CMT2 management into specialty programs. The combination of unmet medical needs and research focus drives its rapid expansion.
- By Severity
On the basis of severity, the CMT disease market is segmented into mild-moderate and moderate-to-severe. The moderate-to-severe segment dominated the market due to higher functional impairments and complex treatment requirements. Patients often experience significant muscle weakness, sensory deficits, and mobility issues that require multidisciplinary care. Hospitals and specialty clinics offer integrated management programs combining medication, orthopedic devices, and physiotherapy. For instance, tendon transfer surgeries and supportive braces are commonly used in this segment. Long-term follow-up and rehabilitation increase healthcare resource utilization, reinforcing market dominance. The severity and complexity of these cases ensure higher adoption of advanced therapies and clinical trials.
The mild-to-moderate segment is expected to witness the fastest growth rate from 2025 to 2032, driven by early-stage interventions and increased disease awareness. Patients with mild symptoms benefit from early pharmacological treatment, assistive devices, and lifestyle modifications. For instance, wearable monitoring devices help track symptom progression and optimize therapy plans. Government and advocacy programs promoting early diagnosis are accelerating adoption. The focus on slowing disease progression and improving quality of life drives rapid growth. Hospitals and clinics are expanding services targeting mild-to-moderate cases. This proactive approach supports higher patient engagement and therapy uptake.
- By Drug Type
On the basis of drug type, the market is segmented into branded and generics. The branded drugs segment dominated the market due to extensive clinical validation, high efficacy, and orphan drug incentives. These drugs target underlying disease mechanisms and offer novel therapeutic options. For instance, ADX-71441 and other candidates are primarily branded drugs under late-stage clinical trials. Hospitals and specialty clinics prefer branded drugs for their proven safety profiles. The market dominance is reinforced by strong R&D pipelines and regulatory exclusivity. High adoption rates in developed regions further solidify their leading position. Branded drugs also allow premium pricing, supporting sustained revenue generation.
The generics segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by patent expirations of branded therapies and rising demand for affordable treatment. Generic drugs offer comparable efficacy and safety at lower costs, expanding patient accessibility. For instance, healthcare providers are increasingly prescribing generics to manage budget constraints. Online and retail pharmacies facilitate easy distribution and adoption of generics. Cost containment policies in developing countries further boost demand. Growing awareness and patient preference for affordable options accelerate market penetration. The generics segment’s rapid growth reflects a combination of affordability and broader market reach.
- By Treatment Type
On the basis of treatment type, the market is segmented into medication and surgical interventions. The medication segment dominated the market as it remains the primary treatment approach for managing CMT symptoms. Pharmacological therapies address neuropathic pain, muscle weakness, and functional impairments. For instance, anticonvulsants, NSAIDs, and antidepressants are widely used to improve quality of life. Hospitals and specialty clinics administer medications as part of standard care plans. Long-term treatment and adherence to clinical guidelines ensure high market share. The availability of multiple drug options for symptom management strengthens its dominance. Medication remains the first-line therapy in most CMT cases.
The surgical interventions segment is expected to witness the fastest growth rate from 2025 to 2032, fueled by advancements in orthopedic and neurosurgical procedures. Surgeries such as tendon transfers, osteotomies, and joint corrections help restore mobility and reduce deformities. For instance, patients with severe foot drop or hand deformities benefit from surgical correction combined with physiotherapy. The adoption of minimally invasive techniques improves recovery times and outcomes. Increasing awareness of surgical benefits is driving adoption in specialty clinics. Growth is supported by clinical evidence demonstrating improved functional independence. Rapidly expanding access to surgical interventions contributes to segment growth.
- By Product Type
On the basis of product type, the market is segmented into ADX-71441, AFC-5128, and Others. The ADX-71441 segment dominated the market due to its advanced clinical development stage and potential to modify disease progression. For instance, trials focus on improving outcomes in CMT1 patients. Hospitals and research centers are prioritizing this product due to its orphan drug status and strong therapeutic potential. The dominance is further supported by high R&D investment and regulatory incentives. Its mechanism of action addresses underlying pathology, enhancing patient quality of life. Positive trial results are expected to strengthen market leadership. Adoption in specialty clinics and hospitals ensures consistent revenue growth.
The AFC-5128 segment is expected to witness the fastest growth rate from 2025 to 2032, driven by precision medicine targeting genetic mutations in CMT. For instance, AFC-5128 is being evaluated across multiple subtypes, expanding its potential patient base. The rising interest in personalized therapy accelerates clinical adoption. Specialty clinics are increasingly offering AFC-5128 in trial settings. Positive outcomes could lead to rapid regulatory approval and commercialization. Growing patient awareness and advocacy initiatives further drive uptake. Market expansion is supported by ongoing investment in clinical trials and novel therapy development.
- By Population
On the basis of population, the market is segmented into children and adults. The adult segment dominated the market due to the higher prevalence of CMT onset during adolescence and adulthood. Adults require long-term symptom management and supportive care, driving higher therapy adoption. For instance, clinical trials often focus on adult cohorts to evaluate efficacy and safety. Hospitals and specialty clinics provide integrated care for adults, including medication, rehabilitation, and surgical interventions. Long-term monitoring and follow-up increase market share. Adults represent the largest patient population, reinforcing dominance in revenue generation. Established treatment protocols and accessibility ensure sustained adoption.
The children segment is expected to witness the fastest growth rate from 2025 to 2032, driven by early diagnosis and intervention. Pediatric CMT cases are being identified more frequently due to genetic screening and awareness programs. For instance, early-stage interventions can prevent progression and improve developmental outcomes. Specialized pediatric rehabilitation services and assistive devices are gaining adoption. Advocacy programs for rare childhood diseases are increasing treatment accessibility. The market is growing as clinicians focus on preventive care and tailored therapies. Rising awareness among parents and healthcare providers supports rapid expansion.
- By End User
On the basis of end user, the market is segmented into hospitals, specialty clinics, and others. The hospitals segment dominated the market as the primary care providers for comprehensive CMT management. Hospitals provide diagnostics, medication, rehabilitation, and surgical interventions under one roof. For instance, tertiary hospitals offer multidisciplinary programs integrating neurology, orthopedics, and physiotherapy. Hospitals also participate in clinical trials, giving patients early access to novel therapies. Large patient volumes and specialized care infrastructure ensure dominant market share. Collaboration with pharmaceutical companies supports adoption of branded drugs. Hospital networks play a key role in patient education and disease management.
The specialty clinics segment is expected to witness the fastest growth rate from 2025 to 2032, driven by focused neuromuscular care and personalized therapies. Clinics provide genetic counseling, electrophysiological testing, and individualized rehabilitation plans. For instance, specialty centers are increasingly offering trial access to AFC-5128 and ADX-71441. Targeted care improves patient outcomes and satisfaction. Rising demand for specialized attention for rare disorders fuels growth. Clinics are expanding in urban and semi-urban regions to meet patient needs. Personalized approaches and early interventions contribute to rapid adoption.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacies, retail pharmacies, online pharmacies, and others. The hospital pharmacies segment dominated the market due to their critical role in dispensing specialized CMT medications and facilitating clinical trial access. For instance, ADX-71441 and other branded drugs are primarily distributed through hospital pharmacies. Hospitals provide integrated care including drug administration and follow-up. Clinical collaborations support trial participation and patient adherence. Large-scale pharmacy networks ensure reliable supply for chronic management. Hospital pharmacies are preferred for controlled distribution and monitoring of advanced therapies. Their infrastructure supports long-term therapy management.
The online pharmacies segment is expected to witness the fastest growth rate from 2025 to 2032, fueled by convenience, digital adoption, and accessibility. Patients increasingly prefer online delivery of generics, supportive care products, and trial medications. For instance, telemedicine and e-prescriptions complement online pharmacy distribution. Online platforms reduce access barriers for patients in remote areas. Rising e-commerce penetration and home delivery services accelerate adoption. The convenience and affordability of online pharmacies drive rapid market growth. Healthcare providers are increasingly collaborating with online pharmacies to enhance patient reach.
Charcot Marie Tooth Disease Market Regional Analysis
- North America dominated the CMT disease market with the largest revenue share of 44.42% in 2024. This dominance is attributed to a strong research ecosystem, major companies conducting clinical trials, and early adoption of novel therapies. The high prevalence of CMT in the region further contributes to its market leadership
- Patients and healthcare providers in the region highly value access to innovative therapies, early diagnosis through genetic testing, and specialized care programs at hospitals and neuromuscular clinics
- This widespread adoption is further supported by high healthcare expenditure, robust clinical trial activity, and strong government and private funding for rare disease research, establishing North America as a leading market for CMT therapies
U.S. Charcot Marie Tooth Disease Market Insight
The U.S. CMT disease market captured the largest revenue share of 82% in 2024 within North America, fueled by the well-established healthcare infrastructure and high awareness of rare genetic disorders. Patients and healthcare providers increasingly prioritize early diagnosis through advanced genetic testing and timely intervention to manage disease progression. The growing adoption of innovative therapies, including ADX-71441 and AFC-5128, combined with access to specialized neuromuscular clinics, further propels the market. Moreover, the U.S. benefits from extensive clinical trial activity and government and private funding for rare disease research, significantly contributing to market expansion.
Europe Charcot-Marie-Tooth Disease Market Insight
The Europe CMT disease market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by advanced healthcare systems and supportive regulatory frameworks for orphan drugs. Increasing awareness of rare genetic disorders, along with early diagnostic programs, is fostering market growth. European patients are drawn to specialized treatment centers, multidisciplinary care programs, and rehabilitation facilities that improve quality of life. The region is experiencing significant growth across residential, hospital, and specialty clinic applications, with therapies being incorporated into both new treatment protocols and existing patient management systems.
U.K. Charcot-Marie-Tooth Disease Market Insight
The U.K. CMT disease market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising awareness of rare neuromuscular disorders and the increasing availability of advanced genetic testing. In addition, the demand for early intervention and personalized treatment plans is encouraging hospitals and specialty clinics to adopt novel therapies. The U.K.’s strong healthcare infrastructure, coupled with robust research initiatives and access to clinical trials, is expected to continue stimulating market growth. The focus on preventive care, rehabilitation, and patient education further supports adoption of CMT therapies across the country.
Germany Charcot-Marie-Tooth Disease Market Insight
The Germany CMT disease market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of genetic disorders and the demand for technologically advanced diagnostic and treatment solutions. Germany’s well-developed healthcare system, combined with emphasis on innovation, promotes adoption of novel CMT therapies in hospitals and specialty clinics. Multidisciplinary care programs, integration of advanced rehabilitation, and access to clinical trials are driving adoption. Patients and providers increasingly seek therapies that improve long-term outcomes, making Germany a key market for CMT treatment solutions.
Asia-Pacific Charcot-Marie-Tooth Disease Market Insight
The Asia-Pacific CMT disease market is poised to grow at the fastest CAGR of 23–25% during the forecast period of 2025 to 2032, driven by rising awareness of rare genetic disorders, expanding healthcare infrastructure, and increasing access to specialty clinics in countries such as Japan, China, and India. The region’s growing inclination towards genetic testing, early diagnosis, and multidisciplinary care is driving adoption of CMT therapies. Moreover, as APAC develops capabilities in clinical trials and rare disease research, the accessibility and availability of treatments are improving for a wider patient population.
Japan Charcot-Marie-Tooth Disease Market Insight
The Japan CMT disease market is gaining momentum due to the country’s advanced healthcare system, high patient awareness, and demand for early diagnosis and personalized care. Japanese patients place significant emphasis on integrated care, including genetic testing, rehabilitation, and novel pharmacological interventions. Adoption of therapies such as ADX-71441 is increasing, alongside the integration of multidisciplinary treatment programs in hospitals and specialty clinics. Moreover, Japan’s aging population is such asly to spur demand for therapies that manage disease progression and maintain functional independence in both residential and clinical settings.
India Charcot-Marie-Tooth Disease Market Insight
The India CMT disease market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to the expanding healthcare access, growing awareness of rare diseases, and rising patient population. India is increasingly adopting genetic testing, early intervention programs, and specialized treatment centers to manage CMT effectively. The push towards improved healthcare infrastructure, coupled with availability of both branded and generic therapies, is propelling market growth. Moreover, government initiatives, NGO support, and rising private investment in rare disease research are key factors accelerating the adoption of CMT treatment solutions across the country.
Charcot Marie Tooth Disease Market Share
The Charcot Marie Tooth Disease industry is primarily led by well-established companies, including:
- Actio Biosciences, Inc. (U.S.)
- Alesta Therapeutics (U.S.)
- Augustine Therapeutics (U.K.)
- ADDEX THERAPEUTICS (Switzerland)
- Bristol-Myers Squibb Company (U.S.)
- Cilcare (France)
- Genzyme Corporation (U.S.)
- Helixmith Co., Ltd. (South Korea)
- InFlectis BioScience (France)
- Ionis Pharmaceuticals, Inc. (U.S.)
- MedDay Pharmaceuticals (France)
- NMD Pharma A/S (Denmark)
- Pharnext SA (France)
- Recursion (U.S.)
- Samsara Therapeutics (U.S.)
- Sarepta Therapeutics (U.S.)
- SOM Biotech (Spain)
- Vincent Timmerman (Belgium)
- Zynerba Pharmaceuticals (U.S.)
What are the Recent Developments in Global Charcot Marie Tooth Disease Market?
- In September 2025, the CMT Research Foundation announced funding for Elpida Therapeutics to begin manufacturing ELP-02, a gene therapy targeting CMT4J. This move brings the therapy closer to clinical trials. The funding, totaling USD 1.5 million, will support the production of clinical-grade doses for a Phase I/II trial, with patient treatment expected to commence in early to mid-2026
- In July 2025, the Medical University of South Carolina (MUSC) was designated as the state's first CMT Center of Excellence by the Charcot-Marie-Tooth Association, aiming to enhance patient care and research. This recognition underscores MUSC's commitment to providing expert multidisciplinary care for individuals with CMT
- In May 2025, Augustine Therapeutics initiated a Phase I clinical trial for AGT-100216, a potential disease-modifying therapy for CMT. The first human participant was dosed, marking a significant milestone in CMT treatment development. The trial is a randomized, double-blind, placebo-controlled study designed to evaluate the safety, tolerability, pharmacokinetics, and exploratory pharmacodynamics of oral AGT-100216 in healthy adult volunteers
- In May 2025, the Charcot-Marie-Tooth Association (CMTA) presented a detailed analysis identifying 135 genes and 169 subtypes associated with CMT at the Annual Meeting of the Peripheral Nerve Society. This research enhances understanding of the disease's genetic diversity and underscores the importance of continued genetic discovery
- In October 2024, the Hereditary Neuropathy Foundation (HNF) reported strong results from a study using wearable technology to monitor CMT patients, indicating potential for improved clinical trial outcomes. The study's Phase 2 results are anticipated to be released in late summer 2025, which could further validate the use of wearable tech in CMT research
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.