Global Chemotherapy Induced Peripheral Neuropathy Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
967.73 Million
USD
1,503.28 Million
2021
2029
USD
967.73 Million
USD
1,503.28 Million
2021
2029
| 2022 –2029 | |
| USD 967.73 Million | |
| USD 1,503.28 Million | |
|
|
|
|
Chemotherapy Induced Peripheral Neuropathy Treatment Market Analysis and Size
As per the data provided by the Centers for Disease Control and Prevention (CDC), in the U.S., 1,708,921 new cancer cases were witnessed in 2018 and 599,265 people died of cancer. The demand for chemotherapy has increased worldwide with the increasing burden of cancer. Peripheral neuropathy includes symptoms that result from damage to the peripheral nerves. Chemotherapy and several other medications are used to treat cancer that can cause peripheral neuropathy.
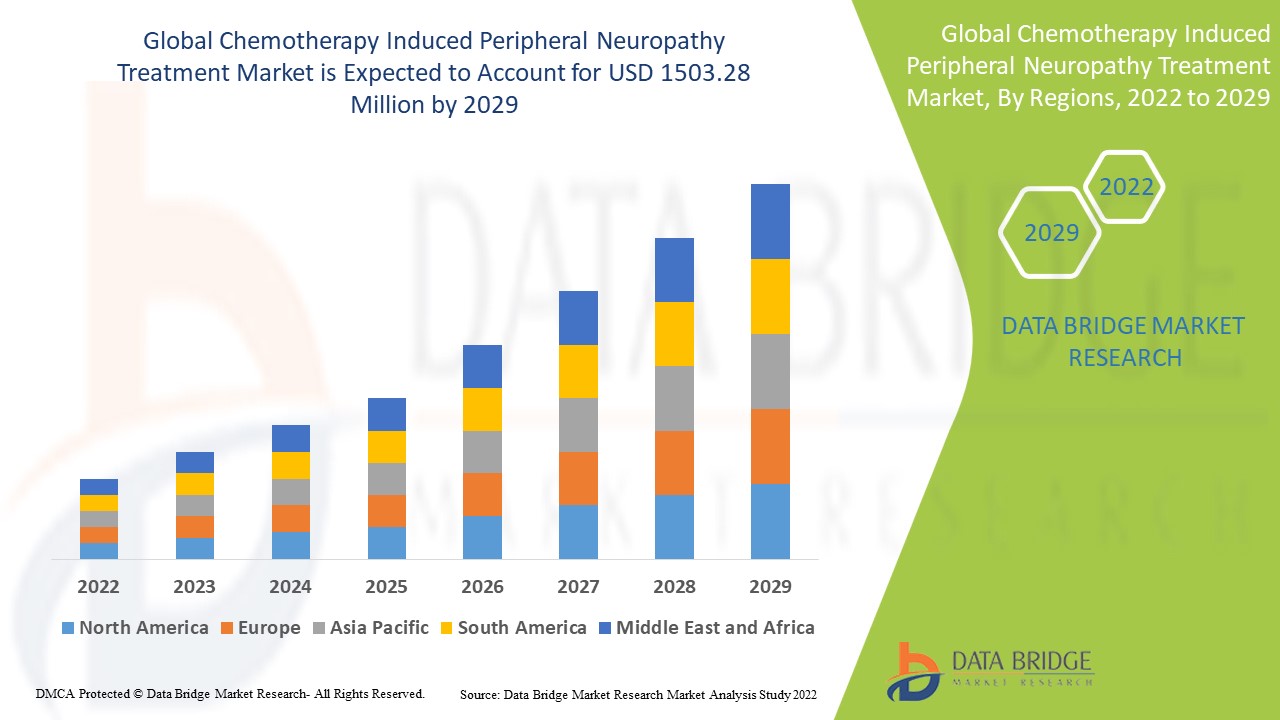
Data Bridge Market Research analyses a growth rate in the global chemotherapy induced peripheral neuropathy treatment market in the forecast period 2022-2029. The expected CAGR of global chemotherapy induced peripheral neuropathy treatment market tend to be around 5.66% in the mentioned forecast period. The market was valued at USD 967.73 million in 2021, and it would grow upto USD 1503.28 million by 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Chemotherapy Induced Peripheral Neuropathy Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Treatment (Medication, Therapy, Others), Drug Class (Nerve Protective Therapy, Anti-Inflammatory Therapy, Neurotransmitter Based Therapy, Antioxidant, Others), Drug Type (Branded, Generic),End-User (Hospitals, Research Institutes, Specialty Clinics), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, online Pharmacies and Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Alexion Pharmaceuticals, Inc. (U.S.), Sanofi (France), Takeda Pharmaceutical Company Limited (Japan), Vertex Pharmaceuticals Incorporated (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Abbott (U.S.), Amgen Inc. (U.S.), Sun Pharmaceuticals Industries Ltd. (India), Amneal Pharmaceuticals LLC. (U.K.), Asahi Kasei Pharma Corporation (Japan), Solasia Pharma K.K. (Japan), Immunepharma.com. (U.S.) |
|
Market Opportunities |
|
Market Definition
Chemotherapy induced peripheral neuropathy is a severe type of clinical condition induced by the number of chemotherapeutics drugs or cytotoxic drugs such as platinum, vinca alkaloids, taxanes, eribulin, epothilones and bortezomib. All these drugs cause altered pathological problem to the neurons. The pathological problems lead to the degeneration of peripheral sensory and motor nerves that can cause patients to have sensory disturbances, balance problems or weakness.
Global Chemotherapy Induced Peripheral Neuropathy Treatment Market Dynamics
Drivers
- Increase in Advanced Treatments
The rising advanced treatment methods are boosting in the market growth. Advanced cancer treatments improve patient survival and surge the number of patients who needs long-term outpatient chemotherapy. More exposure to chemotherapy may result in a rising number of these diseased cases, thus driving the chemotherapy-induced peripheral neuropathy market growth. Thus, it acts as a major driver in the market growth.
- Increasing Prevalence of Cancer
It has been witnessed that for every 100,000 people, 436 new cancer cases were seen and 149 people died of cancer. According to the data published by International Agency for Research on Cancer (IARC), 22,81,658 new cancer cases were witnessed in 2020 in the U.S., where breast cancer was predominant, accounting for 11.1% of all reported cases. This boosts the market growth.
Opportunities
- Increase in Smoking and Lifestyle Changes
Cigarette smoking is one of the leading cause of preventable disease and death in the U.S. It accounts for more than 480,000 deaths every year. In addition to this, the lifestyle changes that are attained by today’s population such as unhealthy lifestyle, poor diet, stress and many such factors altogether contribute to the developing opportunities of the market.
- Growing Initiatives by Market Players
The rising initiatives by several market players are creating in much opportunities for the market growth. For instance, In March 2020, Regenacy Pharmaceuticals announced the closing of a US$ 30 million Series A financing. This initiative was co-led by Cobro Ventures and Taiwania Capital Management Corporation, with the active participation of Yonjin Capital, 3E Bioventures Capital, VIVA Biotech Holdings, and TA YA VENTURE HOLDINGS LIMITED, and other several undisclosed private investors.
Restraints/Challenges
- Lack of skilled professionals
The lack of qualified personnel who are unable to perform these treatments could limit the growth of the global chemotherapy induced peripheral neuropathy treatment market over a forecast period.
- High Cost
The huge expenditure required for the treatment processes hamper the market growth. High costs are associatred with chemotherapy regimens, which somewhat hamper the market's growth.
This global chemotherapy induced peripheral neuropathy treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global chemotherapy induced peripheral neuropathy treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Global Chemotherapy Induced Peripheral Neuropathy Treatment Market
COVID-19 has left a major global public health crisis that has impacted practically every market sector. Its long-term consequences are expected to boost the industry growth during the forecast period.
The load on healthcare facilities and professionals was increasing with the rapid surge in patient numbers. Many hospitals have decided to cancel or postpone procedures. The pandemic had a mixed influence on the global chemotherapy induced peripheral neuropathy treatment market. The pandemic has put a strain on the treatment procedures in general. As a result of the lockdown and travel restrictions, the number of hospitals has decreased and countless drives have been cancelled around the world.
Recent Developments:
- In December 2020, for the development of ricolinostat, Regenacy announced a joint venture with 3E Bioventures in China, which will be further followed by a U.S.-based phase 2 study in patients with painful diabetic peripheral neuropathy and other peripheral neuropathies. This collaboration will evaluate the efficacy and safety of ricolinostat in patients having chemotherapy-induced peripheral neuropathy in China.
Global Chemotherapy Induced Peripheral Neuropathy Treatment Market Scope
The global chemotherapy induced peripheral neuropathy treatment market is segmented on the basis of drug class, drug type, treatment, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Class
- Nerve Protective Therapy
- Anti-Inflammatory Therapy
- Neurotransmitter Based Therapy
- Antioxidant
- Others
Treatment
- Medication
- Therapy
- Others
Drug Type
- Branded
- Generic
End-User
- Hospitals
- Research Institutes
- Specialty Clinics
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacies
- Others
Chemotherapy Induced Peripheral Neuropathy Treatment Market Regional Analysis/Insights
The global chemotherapy induced peripheral neuropathy treatment market is analyzed and market size insights and trends are provided by drug class, drug type, treatment, distribution channel and end-user as referenced above.
The major countries covered in the global chemotherapy induced peripheral neuropathy treatment market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the market in the forecast period due to the presence of high healthcare expenditure and favourable reimbursement policies for the treatment.
Asia-Pacific is expected to have the largest market share over coming years for the chemotherapy-induced peripheral neuropathy treatment market due to the constant rise in cancer incidence with increased demand for cost-efficient therapeutics.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Chemotherapy Induced Peripheral Neuropathy Treatment Market Share Analysis
The global chemotherapy induced peripheral neuropathy treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to global chemotherapy induced peripheral neuropathy treatment market.
Key players operating in the global chemotherapy induced peripheral neuropathy treatment market include:
- Alexion Pharmaceuticals, Inc. (U.S.)
- Sanofi (France)
- Takeda Pharmaceutical Company Limited (Japan)
- Vertex Pharmaceuticals Incorporated (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Abbott (U.S.)
- Amgen Inc. (U.S.)
- Jazz Pharmaceuticals, Inc. (U.K.)
- Asahi Kasei Pharma Corporation (Japan)
- Solasia Pharma K.K. (Japan)
- Immunepharma.com. (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 INDUSTRY INSIGHTS
5.1 PATENT ANALYSIS
5.1.1 PATENT LANDSCAPE
5.1.2 USPTO NUMBER
5.1.3 PATENT EXPIRY
5.1.4 EPIO NUMBER
5.1.5 PATENT STRENGTH AND QUALITY
5.1.6 PATENT CLAIMS
5.1.7 PATENT CITATIONS
5.1.8 PATENT LITIGATION AND LICENSING
5.1.9 FILE OF PATENT
5.1.10 PATENT RECEIVED CONTRIES
5.1.11 TECHNOLOGY BACKGROUND
5.2 DRUG TREATMENT RATE BY MATURED MARKETS
5.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.4 PATIENT FLOW DIAGRAM
5.5 KEY PRICING STRATEGIES
5.6 KEY PATIENT ENROLLMENT STRATEGIES
5.7 INTERVIEWS WITH SPECIALIST
5.8 OTHER KOL SNAPSHOTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATIENT TREATMENT SUCCESS RATES
7 MERGERS AND ACQUISITION
7.1 LICENSING
7.2 COMMERCIALIZATION AGREEMENTS
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yest Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
10 MARKETED DRUG ANALYSIS
10.1 DRUG
10.1.1 BRAND NAME
10.1.2 GENERICS NAME
10.2 THERAPEUTIC INDIACTION
10.3 PHARACOLOGICAL CLASS OD THE DRUG
10.4 DRUG PRIMARY INDICATION
10.5 MARKET STATUS
10.6 MEDICATION TYPE
10.7 DRUG DOSAGES FORM
10.8 DOSAGES AVAILABILITY
10.9 PACKAGING TYPE
10.1 DRUG ROUTE OF ADMINISTRATION
10.11 DOSING FREQUENCY
10.12 DRUG INSIGHT
10.13 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
10.13.1 FORECAST MARKET OUTLOOK
10.13.2 CROSS COMPETITION
10.13.3 THERAPEUTIC PORTFOLIO
10.13.4 CURRENT DEVELOPMENT SCENARIO
11 MARKET ACCESS
11.1 10-YEAR MARKET FORECAST
11.2 CLINICAL TRIAL RECENT UPDATES
11.3 ANNUAL NEW FDA APPROVED DRUGS
11.4 DRUGS MANUFACTURER AND DEALS
11.5 MAJOR DRUG UPTAKE
11.6 CURRENT TREATMENT PRACTICES
11.7 IMPACT OF UPCOMING THERAPY
12 R & D ANALYSIS
12.1 COMPARATIVE ANALYSIS
12.2 DRUG DEVELOPMENTAL LANDSCAPE
12.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
12.4 THERAPEUTIC ASSESSMENT
12.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
13 MARKET OVERVIEW
13.1 DRIVERS
13.2 RESTRAINTS
13.3 OPPORTUNITIES
13.4 CHALLENGES
14 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, SWOT AND DBMR ANALYSIS
15 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, BY TREATMENT TYPE
15.1 OVERVIEW
15.2 MEDICATION
15.2.1 SEROTONIN-NOREPINEPHRINE REUPTAKE INHIBITORS
15.2.1.1. DULOXETINE
15.2.1.2. VENLAFAXINE
15.2.1.3. OTHERS
15.2.2 ANTICONVULSANTS
15.2.2.1. GABAPENTIN
15.2.2.2. PREGABALIN
15.2.2.3. Á2-DELTA LIGANDS
15.2.2.4. OTHERS
15.2.3 ANTIDEPRESSANTS
15.2.3.1. AMITRIPTYLINE
15.2.3.2. NORTRIPTYLINE
15.2.3.3. OTHERS
15.2.4 NARCOTICS/ OPIOIDS
15.2.4.1. TRAMADOL
15.2.4.2. OXYCODONE
15.2.4.3. TAPENTADOL
15.2.4.4. OTHERS
15.2.5 TOPICAL ANALGESICS
15.2.6 STEROIDS
15.2.7 VITAMINS
15.2.8 EMERGING/PIPELINE DRUGS
15.2.9 OTHERS
15.3 THERAPY
15.3.1 CRYOTHERAPY
15.3.2 TRANSCUTANEOUS NERVE STIMULATION (TENS)
15.3.3 OCCUPATIONAL THERAPY
15.3.4 PHYSICAL THERAPY
15.3.5 COMPRESSION THERAPY
15.3.6 OTHERS
15.4 OTHERS
16 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, BY PATIENT TYPE
16.1 OVERVIEW
16.2 MALE
16.3 FEMALE
16.4
17 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, BY DRUG TYPE
17.1 OVERVIEW
17.2 BRANDED
17.3 GENERIC
18 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, BY POPULATION TYPE
18.1 OVERVIEW
18.2 ADULT
18.3 GERIATRICS
18.4 PEDIATRIC
19 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, BY MODE OF PURCHASE
19.1 OVERVIEW
19.2 PRESCRIPTION
19.3 OVER-THE-COUNTER (OTC)
20 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
20.1 OVERVIEW
20.2 ORAL
20.2.1 TABLETS
20.2.2 CAPSULES
20.2.3 OTHERS
20.3 PARENTERAL
20.4 OTHERS
21 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, BY TYPE
21.1 OVERVIEW
21.2 CHRONIC
21.3 SYMMETRIC
21.4 SENSORY NEUROPATHY
21.5 OTHERS
22 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, BY END USER
22.1 OVERVIEW
22.2 HOSPITALS
22.2.1 PUBLIC
22.2.2 PRIVATE
22.3 SPECIALTY CLINICS
22.4 AMBULATORY CARE
22.5 HOME HEALTHCARE
22.6 OTHERS
23 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL
23.1 OVERVIEW
23.2 DIRECT TENDERS
23.3 RETAIL SALES
23.3.1 HOSPITAL PHARMACY
23.3.2 ONLINE PHARAMCY
23.3.3 RETAIL PHARMACY
23.4 OTHERS
24 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, COMPANY LANDSCAPE
24.1 COMPANY SHARE ANALYSIS: GLOBAL
24.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
24.3 COMPANY SHARE ANALYSIS: EUROPE
24.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
24.5 MERGERS & ACQUISITIONS
24.6 NEW PRODUCT DEVELOPMENT & APPROVALS
24.7 EXPANSIONS
24.8 REGULATORY CHANGES
24.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
25 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, BY REGION
Global CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT Market, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
25.1 NORTH AMERICA
25.1.1 U.S.
25.1.2 CANADA
25.1.3 MEXICO
25.2 EUROPE
25.2.1 GERMANY
25.2.2 U.K.
25.2.3 ITALY
25.2.4 FRANCE
25.2.5 SPAIN
25.2.6 RUSSIA
25.2.7 SWITZERLAND
25.2.8 TURKEY
25.2.9 BELGIUM
25.2.10 NETHERLANDS
25.2.11 DENMARK
25.2.12 SWEDEN
25.2.13 POLAND
25.2.14 NORWAY
25.2.15 FINLAND
25.2.16 REST OF EUROPE
25.3 ASIA-PACIFIC
25.3.1 JAPAN
25.3.2 CHINA
25.3.3 SOUTH KOREA
25.3.4 INDIA
25.3.5 SINGAPORE
25.3.6 THAILAND
25.3.7 INDONESIA
25.3.8 MALAYSIA
25.3.9 PHILIPPINES
25.3.10 AUSTRALIA
25.3.11 NEW ZEALAND
25.3.12 VIETNAM
25.3.13 TAIWAN
25.3.14 REST OF ASIA-PACIFIC
25.4 SOUTH AMERICA
25.4.1 BRAZIL
25.4.2 ARGENTINA
25.4.3 REST OF SOUTH AMERICA
25.5 MIDDLE EAST AND AFRICA
25.5.1 SOUTH AFRICA
25.5.2 EGYPT
25.5.3 BAHRAIN
25.5.4 UNITED ARAB EMIRATES
25.5.5 KUWAIT
25.5.6 OMAN
25.5.7 QATAR
25.5.8 SAUDI ARABIA
25.5.9 REST OF MEA
25.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
26 GLOBAL CHEMOTHERAPY INDUCED PERIPHERAL NEUROPATHY TREATMENT MARKET, COMPANY PROFILE
26.1 WEX PHARMACEUTICALS INC.
26.1.1 COMPANY OVERVIEW
26.1.2 REVENUE ANALYSIS
26.1.3 GEOGRAPHIC PRESENCE
26.1.4 PRODUCT PORTFOLIO
26.1.5 RECENT DEVELOPMENTS
26.2 ALGOTX
26.2.1 COMPANY OVERVIEW
26.2.2 REVENUE ANALYSIS
26.2.3 GEOGRAPHIC PRESENCE
26.2.4 PRODUCT PORTFOLIO
26.2.5 RECENT DEVELOPMENTS
26.3 ESTEVE
26.3.1 COMPANY OVERVIEW
26.3.2 REVENUE ANALYSIS
26.3.3 GEOGRAPHIC PRESENCE
26.3.4 PRODUCT PORTFOLIO
26.3.5 RECENT DEVELOPMENTS
26.4 ASAHI KASEI PHARMA
26.4.1 COMPANY OVERVIEW
26.4.2 REVENUE ANALYSIS
26.4.3 GEOGRAPHIC PRESENCE
26.4.4 PRODUCT PORTFOLIO
26.4.5 RECENT DEVELOPMENTS
26.5 TORAY INDUSTRIES, INC.
26.5.1 COMPANY OVERVIEW
26.5.2 REVENUE ANALYSIS
26.5.3 GEOGRAPHIC PRESENCE
26.5.4 PRODUCT PORTFOLIO
26.5.5 RECENT DEVELOPMENTS
26.6 EGETIS THERAPEUTICS
26.6.1 COMPANY OVERVIEW
26.6.2 REVENUE ANALYSIS
26.6.3 GEOGRAPHIC PRESENCE
26.6.4 PRODUCT PORTFOLIO
26.6.5 RECENT DEVELOPMENTS
26.7 MAKSCIENTIFIC, LLC
26.7.1 COMPANY OVERVIEW
26.7.2 REVENUE ANALYSIS
26.7.3 GEOGRAPHIC PRESENCE
26.7.4 PRODUCT PORTFOLIO
26.7.5 RECENT DEVELOPMENTS
26.8 MEDICINOVA, INC.
26.8.1 COMPANY OVERVIEW
26.8.2 REVENUE ANALYSIS
26.8.3 GEOGRAPHIC PRESENCE
26.8.4 PRODUCT PORTFOLIO
26.8.5 RECENT DEVELOPMENTS
26.9 AXOPROTEGO THERAPEUTICS
26.9.1 COMPANY OVERVIEW
26.9.2 REVENUE ANALYSIS
26.9.3 GEOGRAPHIC PRESENCE
26.9.4 PRODUCT PORTFOLIO
26.9.5 RECENT DEVELOPMENTS
26.9.6 COMPANY OVERVIEW
26.9.7 REVENUE ANALYSIS
26.9.8 GEOGRAPHIC PRESENCE
26.9.9 PRODUCT PORTFOLIO
26.9.10 RECENT DEVELOPMENTS
26.1 ENVERIC BIOSCIENCES
26.10.1 COMPANY OVERVIEW
26.10.2 REVENUE ANALYSIS
26.10.3 GEOGRAPHIC PRESENCE
26.10.4 PRODUCT PORTFOLIO
26.10.5 RECENT DEVELOPMENTS
26.11 SOLASIA PHARMA K.K.
26.11.1 COMPANY OVERVIEW
26.11.2 REVENUE ANALYSIS
26.11.3 GEOGRAPHIC PRESENCE
26.11.4 PRODUCT PORTFOLIO
26.11.5 RECENT DEVELOPMENTS
26.12 WINSANTOR, INC
26.12.1 COMPANY OVERVIEW
26.12.2 REVENUE ANALYSIS
26.12.3 GEOGRAPHIC PRESENCE
26.12.4 PRODUCT PORTFOLIO
26.12.5 RECENT DEVELOPMENTS
26.13 OSMOL THERAPEUTICS.
26.13.1 COMPANY OVERVIEW
26.13.2 REVENUE ANALYSIS
26.13.3 GEOGRAPHIC PRESENCE
26.13.4 PRODUCT PORTFOLIO
26.13.5 RECENT DEVELOPMENTS
26.14 KANNALIFE SCIENCES, INC.
26.14.1 COMPANY OVERVIEW
26.14.2 REVENUE ANALYSIS
26.14.3 GEOGRAPHIC PRESENCE
26.14.4 PRODUCT PORTFOLIO
26.14.5 RECENT DEVELOPMENTS
26.15 PERIPHAGEN, INC;
26.15.1 COMPANY OVERVIEW
26.15.2 REVENUE ANALYSIS
26.15.3 GEOGRAPHIC PRESENCE
26.15.4 PRODUCT PORTFOLIO
26.15.5 RECENT DEVELOPMENTS
26.16 ANNJI PHARMACEUTICAL CO. LTD
26.16.1 COMPANY OVERVIEW
26.16.2 REVENUE ANALYSIS
26.16.3 GEOGRAPHIC PRESENCE
26.16.4 PRODUCT PORTFOLIO
26.16.5 RECENT DEVELOPMENTS
26.17 EA PHARMA CO., LTD.
26.17.1 COMPANY OVERVIEW
26.17.2 REVENUE ANALYSIS
26.17.3 GEOGRAPHIC PRESENCE
26.17.4 PRODUCT PORTFOLIO
26.17.5 RECENT DEVELOPMENTS
26.18 SONNET BIOTHERAPEUTICS, INC.
26.18.1 COMPANY OVERVIEW
26.18.2 REVENUE ANALYSIS
26.18.3 GEOGRAPHIC PRESENCE
26.18.4 PRODUCT PORTFOLIO
26.18.5 RECENT DEVELOPMENTS
26.19 NOVAREMED AG
26.19.1 COMPANY OVERVIEW
26.19.2 REVENUE ANALYSIS
26.19.3 GEOGRAPHIC PRESENCE
26.19.4 PRODUCT PORTFOLIO
26.19.5 RECENT DEVELOPMENTS
26.2 REGENACY PHARMACEUTICALS, INC.
26.20.1 COMPANY OVERVIEW
26.20.2 REVENUE ANALYSIS
26.20.3 GEOGRAPHIC PRESENCE
26.20.4 PRODUCT PORTFOLIO
26.20.5 RECENT DEVELOPMENTS
26.21 MAIN LINE HEALTH/ANANDA HEMP, INC
26.21.1 COMPANY OVERVIEW
26.21.2 REVENUE ANALYSIS
26.21.3 GEOGRAPHIC PRESENCE
26.21.4 PRODUCT PORTFOLIO
26.21.5 RECENT DEVELOPMENTS
26.22 PFIZER
26.22.1 COMPANY OVERVIEW
26.22.2 REVENUE ANALYSIS
26.22.3 GEOGRAPHIC PRESENCE
26.22.4 PRODUCT PORTFOLIO
26.22.5 RECENT DEVELOPMENTS
26.23 ELI LILLY AND COMPANY
26.23.1 COMPANY OVERVIEW
26.23.2 REVENUE ANALYSIS
26.23.3 GEOGRAPHIC PRESENCE
26.23.4 PRODUCT PORTFOLIO
26.23.5 RECENT DEVELOPMENTS
26.23.6 COMPANY OVERVIEW
26.23.7 REVENUE ANALYSIS
26.23.8 GEOGRAPHIC PRESENCE
26.23.9 PRODUCT PORTFOLIO
26.23.10 RECENT DEVELOPMENTS
26.24 SANOFI
26.24.1 COMPANY OVERVIEW
26.24.2 REVENUE ANALYSIS
26.24.3 GEOGRAPHIC PRESENCE
26.24.4 PRODUCT PORTFOLIO
26.24.5 RECENT DEVELOPMENTS
26.25 TAKEDA PHARMACEUTICAL COMPANY
26.25.1 COMPANY OVERVIEW
26.25.2 REVENUE ANALYSIS
26.25.3 GEOGRAPHIC PRESENCE
26.25.4 PRODUCT PORTFOLIO
26.25.5 RECENT DEVELOPMENTS
26.26 VERTEX PHARMACEUTICALS INCORPORATED
26.26.1 COMPANY OVERVIEW
26.26.2 REVENUE ANALYSIS
26.26.3 GEOGRAPHIC PRESENCE
26.26.4 PRODUCT PORTFOLIO
26.26.5 RECENT DEVELOPMENTS
26.27 F. HOFFMANN-LA ROCHE LTD
26.27.1 COMPANY OVERVIEW
26.27.2 REVENUE ANALYSIS
26.27.3 GEOGRAPHIC PRESENCE
26.27.4 PRODUCT PORTFOLIO
26.27.5 RECENT DEVELOPMENTS
26.28 ABBOTT
26.28.1 COMPANY OVERVIEW
26.28.2 REVENUE ANALYSIS
26.28.3 GEOGRAPHIC PRESENCE
26.28.4 PRODUCT PORTFOLIO
26.28.5 RECENT DEVELOPMENTS
26.29 AMGEN INC.
26.29.1 COMPANY OVERVIEW
26.29.2 REVENUE ANALYSIS
26.29.3 GEOGRAPHIC PRESENCE
26.29.4 PRODUCT PORTFOLIO
26.29.5 RECENT DEVELOPMENTS
26.3 SUN PHARMACEUTICALS INDUSTRIES LTD.
26.30.1 COMPANY OVERVIEW
26.30.2 REVENUE ANALYSIS
26.30.3 GEOGRAPHIC PRESENCE
26.30.4 PRODUCT PORTFOLIO
26.30.5 RECENT DEVELOPMENTS
26.31 AMNEAL PHARMACEUTICALS LLC.
26.31.1 COMPANY OVERVIEW
26.31.2 REVENUE ANALYSIS
26.31.3 GEOGRAPHIC PRESENCE
26.31.4 PRODUCT PORTFOLIO
26.31.5 RECENT DEVELOPMENTS
27 RELATED REPORTS
28 CONCLUSION
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












