Global Childhood Absence Epilepsy Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
220.43 Million
USD
390.14 Million
2022
2030
USD
220.43 Million
USD
390.14 Million
2022
2030
| 2023 –2030 | |
| USD 220.43 Million | |
| USD 390.14 Million | |
|
|
|
|
Childhood Absence Epilepsy Treatment Market Analysis and Size
Childhood absence epilepsy (CAE) typically begins between the ages of 4 and 8 years, with a peak onset around 6 years of age. It is more common in girls than in boys, with a female-to-male ratio of approximately 2:1. On the basis of available data, the estimated incidence of CAE ranges from 2 to 12 per 100,000 children per year. However, it's important to note that these estimates may vary across different studies and populations.
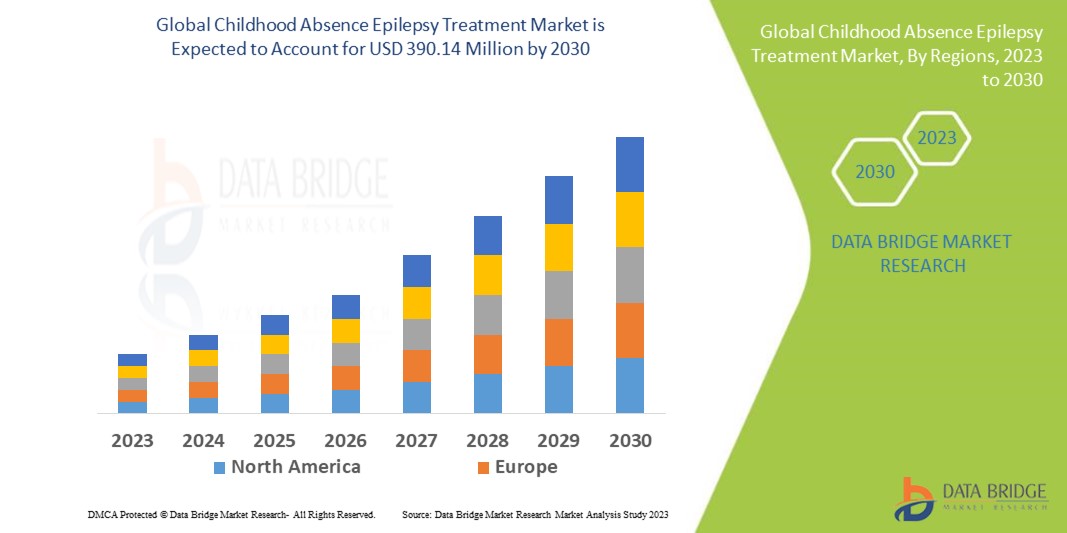
Data Bridge Market Research analyses that the childhood absence epilepsy treatment market which was USD 220.43 million in 2022, would rocket up to USD 390.14 million by 2030, and is expected to undergo a CAGR of 9.7% during the forecast period. This indicates the market value. “Electroencephalogram” dominates the diagnosis segment of the childhood absence epilepsy treatment market owing to the growing demand for better methods for diagnosis and treatment in children. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Childhood Absence Epilepsy Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, and Pricing in USD |
|
Segments Covered |
Drug Type (Cannabidiol Oral Solution, Valproate, Lamotrigine, Ethosuximide, CX-8998, Others), Disease Type (Typical Absence Seizures, Atypical Absence Seizures), Diagnosis (Electroencephalogram, MRI, CT scan), End-Users (Clinic, Hospital, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy) |
|
Countries Covered |
U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, U.A.E., Egypt, Israel, Rest of Middle East and Africa |
|
Market Players Covered |
Jazz Pharmaceuticals, Inc. (Ireland), Pfizer Inc. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), AbbVie Inc. (U.S.), GlaxoSmithKline plc (U.K.), Albany Molecular Research Inc. (AMRI) (U.S.), Johnson & Johnson Private Limited (U.S.), Abbott (U.S.), Bausch Health Companies Inc. (Canada), Biocon (India), Merck KGaA (Germany) and Cadila Pharmaceuticals (India), among others |
|
Market Opportunities |
|
Market Definition
Childhood absence epilepsy (CAE) is a common type of disease which occurs in children and adolescence and caused due to various factors that impact the functioning of brain. Affected children will experience absence seizures which are also known as petit mal seizures. They are the brief episodes of impaired consciousness and each seizure lasts about 10 to 20 seconds and ends abruptly. Childhood absence epilepsy starts between the ages of 4 to 7 years.
Global Childhood Absence Epilepsy Treatment Market Dynamics
Drivers
- Increasing Prevalence of Childhood Absence Epilepsy
Childhood absence epilepsy is one of the most common forms of pediatric epilepsy, with a significant number of children being diagnosed with this condition. The rising prevalence of childhood absence epilepsy drives the demand for effective treatment options, leading to the growth of the market.
- Technological Advancements in Treatment Options
The advancement of technology has led to the development of novel treatment options for childhood absence epilepsy. These include antiepileptic drugs (AEDs) with improved efficacy and safety profiles, as well as non-pharmacological approaches such as neuro-stimulation techniques. Technological advancements in treatment options drive the market by providing more effective and personalized therapies.
Opportunities
- Integration of Digital Health Technologies
Digital health technologies, such as mobile health applications, wearable devices, and remote monitoring systems, offer opportunities for improved management and monitoring of childhood absence epilepsy. These technologies can facilitate real-time data collection, enhance patient engagement, and enable remote access to healthcare providers. The market presents opportunities for the integration of these technologies into treatment approaches, leading to more personalized and accessible care for children with this condition.
- Patient Advocacy and Awareness
Patient advocacy groups play a crucial role in raising awareness about childhood absence epilepsy and advocating for better treatment options and support services. The market presents opportunities for collaboration with these groups to further educate healthcare professionals, caregivers, and the general public about the condition. Increased awareness can lead to earlier diagnosis, improved access to treatment, and better overall outcomes for children with childhood absence epilepsy.
Restraints/Challenges
- Research and Development Gaps
Although there have been advancements in the treatment of childhood absence epilepsy, there is still a need for further research and development. Understanding the underlying mechanisms of the condition, identifying new therapeutic targets, and developing more effective and targeted treatment options are areas that require continued investment and research.
- Limited Access to Specialized Care
Childhood absence epilepsy may require specialized care from pediatric neurologists or epileptologists, who may not be readily available in all regions. Limited access to specialized care can delay diagnosis, treatment initiation, and ongoing management of childhood absence epilepsy. Efforts to improve access to specialized care and telemedicine solutions can help overcome this challenge.
This childhood absence epilepsy treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the childhood absence epilepsy treatment market contact Data Bridge Market Research for an analyst brief, our team will help you make an informed market decision to achieve market growth.
Recent Development
- In May 2020, AbbVie, a research-based global biopharmaceutical firm, announced that it had completed its acquisition of Allergan plc after receiving regulatory approval from all government bodies necessary by the merger agreement, and approval from the Irish High Court. The existing AbbVie growth platform will generate approximately $30 billion in revenues in full year 2020, with combined revenues of roughly $50 billion, thanks to this broad on-market portfolio
Global Childhood Absence Epilepsy Treatment Market Scope
The childhood absence epilepsy treatment market is segmented on the basis of drug type, disease type, diagnosis, end-users and distribution channel. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Type
- Cannabidiol Oral Solution
- Valproate
- Lamotrigine
- Ethosuximide
- CX-8998
- Others
Disease Type
- Typical Absence Seizures
- Atypical Absence Seizures
Diagnosis
- Electroencephalogram
- MRI
- CT scan
End-Users
- Hospitals
- Clinics
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
Global Childhood Absence Epilepsy Treatment Market Regional Analysis/Insights
The global childhood absence epilepsy treatment market is analysed and market size insights and trends are provided by drug type, disease type, diagnosis, end-users and distribution channel as referenced above.
The countries covered in the childhood absence epilepsy treatment market report are U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, U.A.E., Egypt, Israel, Rest of Middle East and Africa.
North America dominates the childhood absence epilepsy treatment market because of the presence of major key players, continuous technological development, ongoing projects, and growing awareness among parents, well-developed healthcare sector and increasing prevalence of disease.
Asia-Pacific is expected to witness significant growth during the forecast period of 2023 to 2030 due to the increasing research and development activities, rising investment in the healthcare sector and growing government support.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as down-stream and up-stream value chain analysis, technical trends, and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, the impact of domestic tariffs, and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed Base and New Technology Penetration
The childhood absence epilepsy treatment market also provides you with a detailed market analysis for every country's growth in healthcare expenditure for capital equipment, installed base of different kinds of products for the childhood absence epilepsy treatment market, the impact of technology using lifeline curves and changes in healthcare regulatory scenarios and their impact on the childhood absence epilepsy treatment market.
Competitive Landscape and Childhood Absence Epilepsy Treatment Market Share Analysis
The childhood absence epilepsy treatment market competitive landscape provides details by competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width, and breadth, application dominance. The above data points provided are only related to the company’s focus related to the childhood absence epilepsy treatment market.
Some of the major players operating in the childhood absence epilepsy treatment market are:
- Jazz Pharmaceuticals, Inc. (Ireland)
- Pfizer Inc. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- AbbVie Inc. (U.S.)
- GlaxoSmithKline plc (U.K.)
- Albany Molecular Research Inc. (AMRI) (U.S.)
- Johnson & Johnson Private Limited (U.S.)
- Abbott (U.S.)
- Bausch Health Companies Inc. (Canada)
- Biocon (India)
- Merck KGaA (Germany)
- Cadila Pharmaceuticals (India)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.