Global Choroideremia Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
3.58 Billion
USD
5.83 Billion
2025
2033
USD
3.58 Billion
USD
5.83 Billion
2025
2033
| 2026 –2033 | |
| USD 3.58 Billion | |
| USD 5.83 Billion | |
|
|
|
|
Choroideremia Treatment Market Size
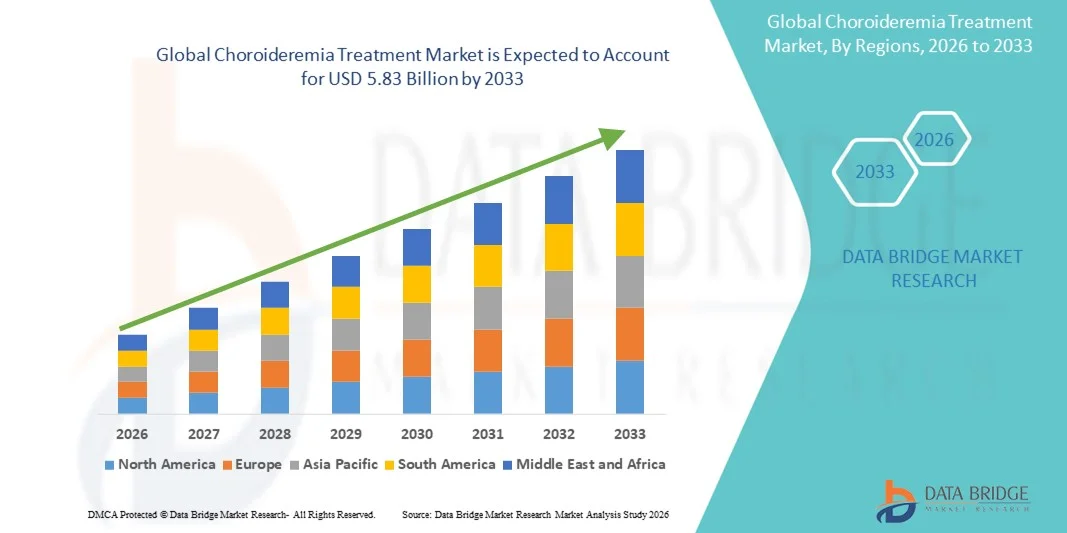
- The global choroideremia treatment market size was valued at USD 3.58 billion in 2025 and is expected to reach USD 5.83 billion by 2033, at a CAGR of 6.28% during the forecast period
- The market growth is largely fueled by the growing adoption of innovative gene therapies, increasing investment in rare disease R&D, and enhanced awareness and diagnosis of this inherited retinal disorder, which are improving accessibility and expanding treatment adoption across key regions
- Furthermore, rising consumer and healthcare demand for effective, targeted treatment solutions combined with expanding healthcare infrastructure and supportive regulatory frameworks is establishing advanced choroideremia treatments as essential interventions in ophthalmology, thereby significantly boosting the industry’s growth
Choroideremia Treatment Market Analysis
- Choroideremia treatments, including gene therapies and supportive interventions, are increasingly vital components of managing this rare inherited retinal disorder due to their potential to slow or halt progressive vision loss and improve patient quality of life
- The escalating demand for choroideremia treatments is primarily fueled by advancements in gene therapy, increased rare disease awareness, and growing investments in ophthalmic R&D, enabling more patients to access innovative treatment options
- North America dominated the choroideremia treatment market with the largest revenue share of 42.3% in 2025, driven by well-established healthcare infrastructure, early adoption of gene therapy solutions, high patient awareness, and the presence of key market players advancing clinical trials and regulatory approvals
- Asia-Pacific is expected to be the fastest-growing region in the choroideremia treatment market during the forecast period due to increasing healthcare access, rising awareness of rare eye disorders, and growing investments in gene therapy research and commercialization
- Gene therapy segment dominated the choroideremia treatment market with a market share of 51.7% in 2025, driven by its potential for long-term efficacy, targeted treatment mechanism, and growing clinical adoption among patients with advanced-stage disease
Report Scope and Choroideremia Treatment Market Segmentation
|
Attributes |
Choroideremia Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Choroideremia Treatment Market Trends
Advancements in Gene Therapy and Personalized Medicine
- A significant and accelerating trend in the global choroideremia treatment market is the rapid development and clinical adoption of gene therapy approaches, aimed at restoring or preserving vision in patients with this rare retinal disorder
- For instance, investigational therapies such as AAV2-based gene therapies are being tested in multiple clinical trials to target the CHM gene defect, showing promising early results in slowing disease progression
- Personalized medicine approaches, including patient-specific dosing and gene-editing strategies, are enabling more precise, effective, and safer treatment options
- The integration of advanced imaging technologies, such as OCT and adaptive optics, with gene therapy trials facilitates more accurate monitoring of retinal structure and function, improving patient outcomes
- This trend towards targeted, individualized treatment is fundamentally reshaping expectations for managing choroideremia, as companies such as Nightstar Therapeutics are developing innovative gene therapy solutions with potential long-term benefits
- The demand for therapies that offer sustained efficacy and tailored treatment strategies is growing rapidly across both rare disease and ophthalmology sectors, driven by unmet patient needs and regulatory support for orphan drugs
- For instance, combination therapies integrating gene therapy with neuroprotective agents are being explored to enhance retinal cell survival and improve long-term vision outcomes
- Increased collaborations between biotech firms and academic institutions are accelerating research innovation and speeding up the development of next-generation therapies for choroideremia
Choroideremia Treatment Market Dynamics
Driver
Increasing Rare Disease Awareness and Technological Advancements
- The rising prevalence of choroideremia and growing awareness of rare inherited retinal disorders are significant drivers of market growth
- For instance, in 2025, Nightstar Therapeutics reported progress in their CHM gene therapy trials, highlighting the potential for improved vision outcomes and expanding patient eligibility for innovative treatments
- Advancements in gene therapy, vector design, and ocular delivery systems are enabling more effective and safer treatment options for patients
- Furthermore, increasing accessibility to genetic testing and early diagnosis allows for timely intervention, improving the prognosis of affected individuals
- The combination of patient awareness, early diagnosis, and cutting-edge technology is positioning gene therapy and other novel treatments as critical solutions in ophthalmology
- For instance, growing teleophthalmology services and remote monitoring technologies are enabling earlier identification of eligible patients, expanding treatment reach
- Supportive orphan drug policies and expedited regulatory approvals in key markets are encouraging investment and development of novel choroideremia therapies
Restraint/Challenge
High Cost and Regulatory Complexity
- The high cost of gene therapies and advanced treatments poses a significant barrier to widespread adoption, particularly in developing regions with limited healthcare funding
- For instance, ongoing pricing negotiations for gene therapy treatments such as Luxturna highlight the financial challenges faced by patients and healthcare systems
- Regulatory hurdles, including complex approval processes for rare disease therapies, can delay market entry and commercialization of innovative treatments
- Furthermore, limited long-term clinical data on safety and efficacy may lead to cautious adoption among clinicians and patients
- Overcoming these challenges requires strategic partnerships, patient assistance programs, and streamlined regulatory pathways to ensure broader access to these life-changing therapies
- While gene therapy holds transformative potential, balancing cost, regulatory compliance, and long-term safety remains critical for sustained market growth
- For instance, reimbursement challenges in certain regions can limit patient access and slow adoption of novel therapies
- Intellectual property disputes and licensing complexities may hinder the pace of innovation and commercialization of new choroideremia treatments
Choroideremia Treatment Market Scope
The market is segmented on the basis of therapy type, route of administration, and distribution channel.
- By Therapy Type
On the basis of therapy type, the choroideremia treatment market is segmented into dorzolamide, topical carbonic anhydrase inhibitor, luxturna (voretigene neparvovec-rzyl), gene therapy, antioxidants, and lutein. The Gene Therapy segment dominated the market with the largest revenue share of 51.7% in 2025, driven by its potential to provide long-term efficacy and address the root cause of the disease by targeting CHM gene mutations. Patients and healthcare providers increasingly prefer gene therapy due to its curative potential compared to conventional supportive treatments. The segment’s growth is supported by ongoing clinical trials, regulatory approvals for Luxturna in certain markets, and heightened awareness among ophthalmologists and rare disease specialists. In addition, gene therapy adoption is encouraged by patient assistance programs and healthcare policies favoring orphan drugs. The precision and personalization of gene therapy allow tailored treatment plans for different patient subgroups, further driving market share. The long-term vision stabilization achieved with gene therapy also increases patient demand, supporting its dominance.
The Luxturna (voretigene neparvovec-rzyl) segment is expected to witness the fastest CAGR from 2026 to 2035, fueled by increasing approvals across multiple regions and expanding clinical application in early-stage patients. Luxturna’s targeted AAV2-based delivery system offers significant improvements in visual function, which attracts both patients and clinicians. The rising prevalence of genetic testing allows more patients to qualify for Luxturna therapy, supporting rapid market uptake. In addition, partnerships between biotech companies and healthcare providers are facilitating broader access, while reimbursement support in developed markets is accelerating adoption. Patient preference for innovative, one-time therapies over lifelong management with antioxidants or topical agents further drives growth. Awareness campaigns and advocacy by rare disease organizations also contribute to its fast adoption.
- By Route of Administration
On the basis of route of administration, the market is segmented into oral, ophthalmic, and intravenous. The Ophthalmic segment dominated the market in 2025, capturing the largest revenue share, due to its direct delivery to the target tissue in the eye, ensuring higher efficacy and reduced systemic side effects. Ophthalmic administration is preferred for both conventional treatments such as Dorzolamide and innovative therapies such as Luxturna, allowing precise dosing and improved patient compliance. Clinicians favor ophthalmic delivery for its localized effect, which reduces potential complications associated with systemic administration. Regulatory approvals for ocular administration routes further support its dominance. Moreover, the route’s integration with surgical procedures, such as subretinal injection for gene therapy, reinforces its prevalence. Ophthalmic formulations are widely available through hospitals and specialized clinics, boosting adoption.
The Intravenous segment is expected to witness the fastest growth during the forecast period, driven by emerging systemic therapies and combination approaches in clinical trials. Intravenous delivery allows experimental gene-editing therapies and biologics to be administered efficiently, targeting retinal cells through systemic circulation. Early-stage research on novel delivery vectors and supportive antioxidants also relies on IV administration. Increasing funding and collaborations for rare retinal disorders accelerate clinical development of IV therapies. The route enables potential multi-organ therapeutic benefits in combination treatments, appealing to advanced-stage patients. Improved patient monitoring and hospital-based administration protocols further facilitate adoption.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacies, retail pharmacies, drugstores, and online pharmacies. The Hospital Pharmacies segment dominated the market with the largest revenue share in 2025, driven by the specialized administration requirements of gene therapies and ophthalmic treatments, which often require clinical supervision. Hospitals provide the necessary infrastructure for subretinal injections, monitoring, and post-treatment care. The preference for hospital pharmacies is reinforced by trained healthcare personnel, ensuring proper handling and storage of sensitive therapies. Partnerships between pharmaceutical companies and hospitals also facilitate direct distribution of rare disease therapies. In addition, hospital pharmacies are pivotal for patient education, follow-up, and insurance coordination. The centralized distribution ensures compliance with regulatory requirements and cold chain management for advanced therapies such as Luxturna.
The Online Pharmacies segment is expected to witness the fastest CAGR from 2026 to 2035, fueled by increasing digital health adoption and patient convenience in accessing medications for supportive treatments such as antioxidants, Dorzolamide, and lutein. Online platforms enable home delivery, subscription models, and easier refills for long-term therapies. The COVID-19 pandemic accelerated the shift toward telemedicine and online pharmacy adoption, which continues to drive growth. Online pharmacies also provide access to remote or underserved regions where hospital or retail pharmacy access is limited. Integration with digital health records and teleconsultation further enhances patient adherence. Marketing and awareness campaigns by rare disease organizations encourage patients to utilize online channels for convenient therapy access.
Choroideremia Treatment Market Regional Analysis
- North America dominated the choroideremia treatment market with the largest revenue share of 42.3% in 2025, driven by well-established healthcare infrastructure, early adoption of gene therapy solutions, high patient awareness, and the presence of key market players advancing clinical trials and regulatory approvals
- Patients and clinicians in the region highly value innovative treatments such as Luxturna and other gene therapy approaches for their potential to halt or slow vision loss, alongside supportive therapies such as Dorzolamide and antioxidants
- This widespread adoption is further supported by strong research and development activities, availability of specialized ophthalmic centers, regulatory incentives for orphan drugs, and high healthcare spending, establishing North America as a key market for both emerging and established choroideremia therapies
U.S. Choroideremia Treatment Market Insight
The U.S. choroideremia treatment market captured the largest revenue share of 79% in 2025 within North America, fueled by early adoption of advanced gene therapies and well-established ophthalmology infrastructure. Patients and healthcare providers are increasingly prioritizing innovative treatments such as Luxturna for halting vision loss and improving quality of life. The growing availability of genetic testing, coupled with specialized retinal centers, further propels the market. Moreover, patient assistance programs and regulatory incentives for orphan drugs are significantly contributing to market expansion. Increasing awareness among clinicians and rare disease organizations is also accelerating treatment adoption.
Europe Choroideremia Treatment Market Insight
The Europe choroideremia treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing awareness of rare retinal disorders and supportive healthcare policies for orphan drugs. The rise in diagnostic capabilities and early intervention programs is fostering adoption of innovative therapies. European patients and physicians are drawn to gene therapy and targeted interventions for their long-term efficacy. The region is experiencing significant growth across hospital-based ophthalmic centers and specialized clinics, with treatments being incorporated into both clinical trials and routine care programs. Government initiatives supporting rare disease research further enhance market growth.
U.K. Choroideremia Treatment Market Insight
The U.K. choroideremia treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing investment in gene therapy research and the growing trend of personalized medicine. Rising awareness among ophthalmologists and patients regarding early diagnosis and treatment options is encouraging uptake. In addition, concerns about progressive vision loss are motivating patients to seek advanced therapeutic interventions. The U.K.’s robust healthcare infrastructure and well-developed clinical trial ecosystem are expected to continue stimulating market growth. Increased access to genetic testing and patient advocacy programs further support adoption of novel therapies.
Germany Choroideremia Treatment Market Insight
The Germany choroideremia treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by advancements in gene therapy and ophthalmic treatment infrastructure. Germany’s well-established healthcare system, coupled with a strong focus on innovation and rare disease management, promotes adoption of advanced therapies. The integration of clinical research, specialized retinal centers, and early diagnostic programs is driving patient access. Furthermore, increasing awareness of rare inherited retinal disorders among clinicians and patients is contributing to market growth. The country’s regulatory framework supporting orphan drugs and personalized medicine solutions aligns with local demand for effective and safe treatments.
Asia-Pacific Choroideremia Treatment Market Insight
The Asia-Pacific choroideremia treatment market is poised to grow at the fastest CAGR of 22% during the forecast period of 2026 to 2035, driven by increasing healthcare access, rising awareness of rare retinal disorders, and growing investments in gene therapy. Countries such as Japan, China, and India are witnessing expanding ophthalmology infrastructure and adoption of advanced therapies. Government initiatives promoting rare disease diagnosis and treatment, along with partnerships between biotech companies and hospitals, are driving patient access. Furthermore, growing patient awareness and affordability improvements are contributing to wider adoption. The region is also emerging as a hub for clinical trials, accelerating innovation and availability of treatments.
Japan Choroideremia Treatment Market Insight
The Japan choroideremia treatment market is gaining momentum due to the country’s advanced healthcare system, high adoption of innovative therapies, and emphasis on early diagnosis. Patients prioritize gene therapy and supportive treatments to maintain vision and quality of life. The integration of genetic testing with ophthalmic care enables timely treatment initiation. Moreover, increasing prevalence of rare retinal disorders and government support for orphan drugs is fueling market growth. Clinical research collaborations and hospital-based specialty centers further contribute to expanding treatment access. Aging population and rising healthcare awareness are such asly to spur demand in both residential and clinical settings.
India Choroideremia Treatment Market Insight
The India choroideremia treatment market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to increasing awareness of rare diseases, growing ophthalmology infrastructure, and improving access to advanced therapies. India is witnessing rising adoption of gene therapy and supportive treatments in specialized clinics and hospitals. Government initiatives supporting rare disease management and increasing availability of genetic testing are key factors driving market growth. Expanding middle-class population and increasing healthcare affordability are further supporting adoption. Partnerships between global biotech companies and local healthcare providers are improving access to innovative therapies. Rising patient awareness campaigns and advocacy programs also contribute to market expansion.
Choroideremia Treatment Market Share
The Choroideremia Treatment industry is primarily led by well-established companies, including:
- MeiraGTx Limited (U.K.)
- REGENXBIO (U.S.)
- GenSight Biologics (France)
- Editas Medicine (U.S.)
- Spark Therapeutics (U.S.)
- Sangamo Therapeutics (U.S.)
- CRISPR Therapeutics AG (Switzerland)
- Nanoscope Therapeutics, Inc. (U.S.)
- 4D Molecular Therapeutics (U.S.)
- ProQR Therapeutics N.V. (Netherlands)
- Horama SA (France)
- Homology Medicines, Inc. (U.S.)
- Beacon Therapeutics (U.S.)
- Eyevensys (Switzerland)
- Visgenx (U.S.)
- Atsena Therapeutics Inc. (U.S.)
- Coave Therapeutics (U.S.)
- Neurophth Therapeutics (China)
- Johnson & Johnson Services, Inc. (U.S.)
- Ocugen, Inc. (U.S.)
What are the Recent Developments in Global Choroideremia Treatment Market?
- In October 2025, DelveInsight and other industry pipeline insights highlighted expanding clinical research and multi‑modal approaches (gene therapy, gene editing, RNA‑based therapies, optogenetics) advancing through preclinical and clinical stages for choroideremia, signaling broadening therapeutic research activity
- In June 2025, The Choroideremia Research Foundation awarded six new global research grants to accelerate vision science and treatment innovation in choroideremia, funding projects including CRISPR‑based gene editing and long‑term gene therapy follow‑up studies
- In January 2025, Beacon Therapeutics received FDA Regenerative Medicine Advanced Therapy (RMAT) designation for its investigational gene therapy laru‑zova (AGTC‑501), an important regulatory milestone that provides expedited development and review support although this therapy targets a related inherited retinal disease such designations reflect the advancing regulatory environment and precedent for gene therapies for rare retinal disorders relevant to choroideremia treatment progress
- In October 2023, A randomized Phase 3 clinical trial publication reported on subretinal timrepigene emparvovec in adult men with choroideremia, providing peer‑reviewed data on safety and efficacy outcomes, which informs future design of gene therapy approaches
- In June 2021, Biogen announced topline results from its Phase 3 STAR study of timrepigene emparvovec (BIIB111/AAV2‑REP1) for choroideremia, reporting that the gene therapy did not meet its primary endpoint, marking a critical inflection in clinical development strategy for CHM gene therapies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.