Global Cowdens Disease Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.70 Billion
USD
2.76 Billion
2024
2032
USD
1.70 Billion
USD
2.76 Billion
2024
2032
| 2025 –2032 | |
| USD 1.70 Billion | |
| USD 2.76 Billion | |
|
|
|
|
Cowden’s Disease Treatment Market Size
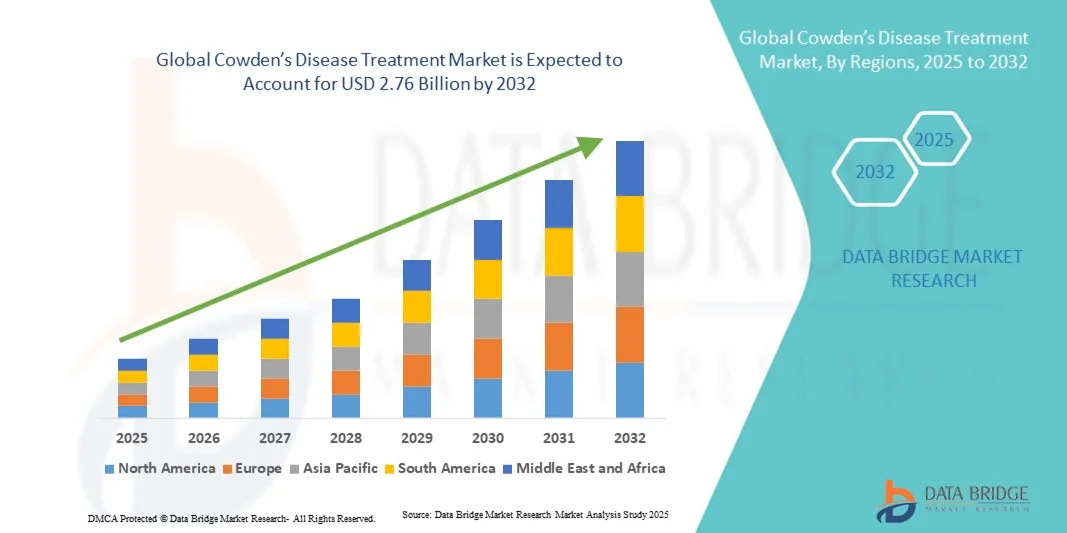
- The global Cowden’s disease treatment market size was valued at USD billion in 2024 and is expected to reach USD 2.76 billion by 2032, at a CAGR of 6.25% during the forecast period
- The market growth is largely fueled by increasing awareness of Cowden’s disease, advancements in genetic diagnostics, and the expanding focus on targeted therapies aimed at PTEN gene mutations, which play a crucial role in disease progression
- Furthermore, the rising demand for early detection methods, personalized treatment approaches, and improved healthcare infrastructure for managing rare genetic disorders is establishing Cowden’s disease treatments as a key area of clinical innovation. These converging factors are accelerating the adoption of novel therapeutic solutions, thereby significantly boosting the industry’s growth.
Cowden’s Disease Treatment Market Analysis
- Cowden’s disease treatment focuses on managing benign and malignant tumor risks associated with PTEN gene mutations through genetic counseling, surveillance, targeted therapies, and surgical interventions. These treatment approaches are becoming increasingly vital within the rare disease and oncology sectors due to growing emphasis on precision medicine and early genetic screening
- The escalating demand for Cowden’s disease treatment is primarily fuelled by increasing awareness of rare genetic disorders, advancements in molecular diagnostics, and expanding access to genetic testing and personalized therapy programs across healthcare systems
- North America dominated the Cowden’s disease treatment market with the largest revenue share of 41.8% in 2024, attributed to well-established healthcare infrastructure, strong research funding for genetic disorders, and the presence of key biopharmaceutical companies investing in rare disease therapeutics. The U.S. continues to lead due to rising clinical trials focused on PTEN-related syndromes and increased patient enrolment in precision oncology initiatives
- Asia-Pacific is expected to be the fastest-growing region in the Cowden’s disease treatment market during the forecast period, driven by improved diagnostic accessibility, government initiatives for rare disease management, and expanding awareness programs in countries such as Japan, China, and India
- The targeted therapy segment dominated the Cowden’s disease treatment market with a market share of 45.2% in 2024, supported by the growing use of mTOR inhibitors and other pathway-specific drugs designed to manage tumor development associated with PTEN mutations
Report Scope and Cowden’s Disease Treatment Market Segmentation
|
Attributes |
Cowden’s Disease Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Cowden’s Disease Treatment Market Trends
Advancements in Precision Medicine and Genetic Diagnostics
- A significant and accelerating trend in the global Cowden’s disease treatment market is the deepening integration of precision medicine and advanced genetic diagnostics for early detection and individualized patient management. This shift is transforming how clinicians identify and treat PTEN gene mutation–associated syndromes
- For instance, advancements in next-generation sequencing (NGS) platforms enable clinicians to detect PTEN mutations with higher accuracy and speed, facilitating more effective monitoring and treatment planning for patients at risk of multiple tumor types
- Integration of molecular profiling tools allows healthcare providers to predict disease progression, personalize treatment protocols, and monitor therapeutic responses. For instance, laboratories are increasingly using multiplex genetic panels to identify overlapping hereditary cancer syndromes in patients with Cowden’s disease
- The combination of molecular diagnostics with AI-driven predictive analytics supports earlier intervention and improved outcomes through data-backed clinical decisions. For instance, emerging AI tools assist in interpreting genetic variants and correlating them with clinical manifestations in PTEN-related disorders
- This trend toward personalized and predictive treatment strategies is redefining patient management in hereditary cancer syndromes. Consequently, companies such as Invitae and Ambry Genetics are expanding their PTEN-specific testing portfolios to improve diagnostic precision and care planning
- The demand for integrated diagnostic and therapeutic solutions is growing rapidly across both developed and emerging markets, as patients and providers increasingly prioritize precision-driven, evidence-based approaches for rare genetic disease management
Cowden’s Disease Treatment Market Dynamics
Driver
Growing Awareness and Advancements in Genetic Testing Technologies
- The increasing global awareness of hereditary cancer syndromes, along with advancements in molecular diagnostics and genetic testing technologies, is a major driver of Cowden’s disease treatment market growth
- For instance, in March 2024, Thermo Fisher Scientific introduced a new NGS-based hereditary cancer panel that includes PTEN mutation analysis, enabling faster and more comprehensive diagnosis of Cowden’s syndrome
- As healthcare systems emphasize early detection and risk assessment, patients are increasingly undergoing genetic screening for PTEN mutations, allowing clinicians to implement preventive and therapeutic interventions more effectively
- Furthermore, the rising number of genetic counseling centers and research collaborations is enhancing access to early diagnosis and care, encouraging greater adoption of precision oncology strategies among medical professionals
- The integration of advanced genetic tests into clinical workflows, coupled with government support for rare disease initiatives, is propelling the expansion of Cowden’s disease treatment options globally, especially in high-income regions with advanced healthcare infrastructure
- For instance, partnerships between biopharmaceutical companies and diagnostic laboratories are enabling faster commercialization of PTEN-focused assays, improving turnaround times and diagnostic accuracy
- The growing role of digital health platforms and tele-genetic counseling services is expanding patient access to Cowden’s disease testing and follow-up care, particularly in underserved and remote regions
Restraint/Challenge
High Cost of Genetic Testing and Limited Clinical Awareness
- The relatively high cost of advanced genetic testing and limited clinical awareness about Cowden’s disease remain key challenges hindering the market’s widespread adoption and timely diagnosis
- For instance, genetic testing for PTEN mutations can cost several hundred to thousands of dollars, restricting accessibility in low- and middle-income countries with limited insurance coverage for rare diseases
- The rarity of Cowden’s syndrome and overlap of its symptoms with other hereditary conditions often lead to misdiagnosis or delayed detection, reducing opportunities for early intervention and effective management
- Furthermore, limited clinician familiarity with PTEN-related disorders restricts referral rates for genetic testing, slowing market expansion and patient access to appropriate therapeutic care
- Overcoming these barriers through government reimbursement initiatives, clinician education, and partnerships between diagnostic firms and healthcare providers will be crucial to improving diagnosis rates and market penetration
- While testing technologies are becoming more affordable, the perceived cost and lack of specialized expertise can still deter early diagnosis, particularly in regions with limited genetic infrastructure or rare disease funding
- The fragmented reimbursement landscape for rare diseases poses additional challenges for patients seeking coverage for diagnostic tests and long-term surveillance, impeding overall market accessibility and growth
Cowden’s Disease Treatment Market Scope
The market is segmented on the basis of site, treatment, and end user.
- By Site
On the basis of site, the Cowden’s disease treatment market is segmented into breast, thyroid, colorectal, kidney, and skin. The breast segment dominated the market with the largest revenue share in 2024, primarily due to the high prevalence of breast lesions and malignancies associated with PTEN gene mutations among Cowden’s disease patients. Regular mammography, MRI screening, and preventive mastectomy options have increased awareness and early diagnosis, driving market growth. Furthermore, continuous advancements in targeted hormonal and surgical therapies have improved patient outcomes in managing breast cancer risks. The availability of multidisciplinary oncology teams and genetic counseling for high-risk patients further enhances the dominance of this segment. The integration of molecular diagnostics with preventive care strategies continues to reinforce the clinical importance of breast-focused treatment in Cowden’s disease.
The thyroid segment is anticipated to witness the fastest growth from 2025 to 2032, driven by rising incidence of thyroid nodules and cancers in PTEN mutation carriers. Increasing adoption of fine-needle aspiration biopsies, ultrasound monitoring, and radioiodine therapy supports early and effective management of thyroid abnormalities. In addition, growing clinical emphasis on molecular profiling of thyroid lesions for precision diagnosis is accelerating demand. The presence of improved diagnostic imaging systems and the inclusion of thyroid surveillance in Cowden’s syndrome management protocols are further propelling segment growth. With expanding awareness and research collaborations targeting thyroid cancer prevention, this segment is poised for substantial expansion.
- By Treatment
On the basis of treatment, the market is segmented into genetic testing, chemotherapy, targeted therapy, surgery & radiation therapy, and hormone therapy. The targeted therapy segment dominated the Cowden’s disease treatment market with a market share of 45.2% in 2024, driven by increasing adoption of precision medicine approaches and the growing focus on PTEN pathway modulation. The use of mTOR inhibitors, PI3K/Akt pathway blockers, and other molecularly targeted drugs has enhanced treatment efficacy while reducing systemic toxicity. Expanding clinical trial activity and rising approvals for orphan drugs addressing rare genetic disorders are strengthening the dominance of this segment. Furthermore, integration of genomic profiling into treatment planning enables clinicians to deliver personalized and adaptive therapies. Strategic collaborations between research institutions and biopharmaceutical companies are also fueling innovation in this space, ensuring sustained leadership of targeted therapy in Cowden’s disease management.
The genetic testing segment is expected to witness the fastest CAGR from 2025 to 2032, as early and accurate PTEN mutation detection continues to serve as the foundation for diagnosis and disease management. Increasing adoption of next-generation sequencing (NGS) and multiplex gene panels has improved testing precision, while the expansion of rare disease awareness programs has accelerated patient screening rates. Partnerships between diagnostic firms and healthcare providers are making testing more accessible and affordable across major healthcare markets. Moreover, advancements in AI-driven variant interpretation tools are streamlining PTEN mutation analysis and guiding personalized therapeutic strategies. As healthcare systems increasingly prioritize proactive genetic screening, the genetic testing segment is poised for significant growth.
- By End User
On the basis of end user, the market is segmented into hospitals and clinics. The hospital segment dominated the market in 2024, driven by the availability of advanced diagnostic infrastructure, multidisciplinary teams, and access to complex surgical and radiotherapy procedures. Hospitals often serve as referral centers for rare disease management and are equipped to handle comprehensive genetic testing, counseling, and long-term patient monitoring. Increasing government and institutional funding for rare disease research has strengthened hospital-based diagnostic programs. In addition, the integration of genetic data into hospital electronic health records (EHRs) enhances care coordination and follow-up. The strong presence of specialized oncology and endocrinology units in tertiary hospitals supports continued dominance of this segment.
The clinics segment is anticipated to grow at the fastest rate during the forecast period, propelled by the decentralization of genetic testing services and the rise of outpatient rare disease management. Clinics offering specialized genetic counseling and telemedicine services are improving patient accessibility, especially in semi-urban and remote areas. Growing partnerships between private clinics and diagnostic laboratories are enabling cost-effective testing and follow-up programs. Furthermore, the increasing demand for personalized care and convenience among patients drives clinic-based treatment adoption. With expanding clinical networks and adoption of digital health tools, clinics are emerging as a key touchpoint for early diagnosis and ongoing Cowden’s disease management.
Cowden’s Disease Treatment Market Regional Analysis
- North America dominated the Cowden’s disease treatment market with the largest revenue share of 41.8% in 2024, attributed to well-established healthcare infrastructure, strong research funding for genetic disorders, and the presence of key biopharmaceutical companies investing in rare disease therapeutics
- Patients in the region increasingly rely on comprehensive genetic testing, targeted therapy options, and multidisciplinary care centers offering personalized management of PTEN mutation–related conditions
- This widespread adoption is further supported by high healthcare spending, well-established reimbursement frameworks, and growing collaboration between research institutions and biopharmaceutical companies, positioning North America as the leading hub for innovation and clinical advancement in Cowden’s disease treatment
U.S. Cowden’s Disease Treatment Market Insight
The U.S. Cowden’s disease treatment market captured the largest revenue share of 80.2% in 2024 within North America, fueled by advanced healthcare infrastructure and strong adoption of precision medicine. Increasing awareness of hereditary cancer syndromes and the widespread availability of PTEN genetic testing are driving market growth. The U.S. also leads in clinical research, with numerous trials evaluating targeted therapies such as mTOR inhibitors and PI3K pathway modulators. Moreover, robust funding for rare disease programs and collaborations between research institutions and biopharmaceutical companies further support market expansion.
Europe Cowden’s Disease Treatment Market Insight
The Europe Cowden’s disease treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by the rising prevalence of rare genetic disorders and the growing implementation of national genomic medicine programs. Increasing adoption of precision diagnostics and early detection initiatives across the U.K., Germany, France, and Italy supports market development. European healthcare systems emphasize comprehensive genetic counseling and multidisciplinary care, enhancing patient outcomes. The presence of well-established academic and clinical research networks is further propelling the regional market’s growth.
U.K. Cowden’s Disease Treatment Market Insight
The U.K. Cowden’s disease treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the country’s emphasis on genetic screening and rare disease research. The National Health Service (NHS) Genomic Medicine Service has expanded PTEN testing accessibility, enabling earlier diagnosis and treatment intervention. Rising public health awareness, government support for rare disease registries, and the presence of leading genomic research institutions contribute to steady market growth. In addition, increasing collaboration between hospitals and diagnostic laboratories enhances nationwide diagnostic reach and treatment adoption.
Germany Cowden’s Disease Treatment Market Insight
The Germany Cowden’s disease treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by the country’s robust biotechnology sector and rising focus on hereditary cancer diagnostics. Strong investments in molecular pathology and genomics research underpin advancements in PTEN mutation testing. Moreover, Germany’s comprehensive healthcare coverage ensures better access to genetic counseling and long-term surveillance programs. Increasing integration of precision oncology and patient-specific therapy in clinical practice continues to drive market growth and strengthen Germany’s position in the European Cowden’s disease treatment landscape.
Asia-Pacific Cowden’s Disease Treatment Market Insight
The Asia-Pacific Cowden’s disease treatment market is poised to grow at the fastest CAGR of 23.6% during the forecast period of 2025 to 2032, driven by increasing adoption of genetic testing, expanding healthcare infrastructure, and growing awareness of hereditary disorders in countries such as Japan, China, and India. Governments across the region are investing in rare disease research and public health genomics initiatives. Furthermore, improving affordability of molecular testing and expanding collaborations between Asian hospitals and global biotech firms are enhancing diagnostic accessibility. As the region continues to digitalize healthcare, precision treatment adoption for Cowden’s disease is accelerating rapidly.
Japan Cowden’s Disease Treatment Market Insight
The Japan Cowden’s disease treatment market is gaining momentum due to the country’s advanced genomic research capabilities, high healthcare standards, and rapid clinical adoption of molecular diagnostics. Growing prevalence of PTEN-related cancer syndromes and government-backed rare disease initiatives are boosting market awareness. For instance, integration of PTEN genetic screening into national cancer prevention programs supports early intervention. Moreover, Japan’s focus on AI-based variant interpretation tools and personalized therapy development further strengthens its role as a leading market in Asia-Pacific.
India Cowden’s Disease Treatment Market Insight
The India Cowden’s disease treatment market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to rising healthcare investments, growing diagnostic awareness, and expanding genomic testing facilities. India’s participation in global rare disease research networks and initiatives such as the National Policy for Rare Diseases is enhancing access to PTEN testing. In addition, increasing private sector involvement, availability of affordable diagnostic services, and growing patient support organizations are driving market growth. The surge in personalized medicine adoption and the presence of skilled genetic counselors further reinforce India’s strong market position.
Cowden’s Disease Treatment Market Share
The Cowden’s Disease Treatment industry is primarily led by well-established companies, including:
- Myriad Genetics (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Illumina, Inc. (U.S.)
- Quest Diagnostics Incorporated (U.S.)
- Labcorp (U.S.)
- Novartis AG (Switzerland)
- AstraZeneca (U.K.)
- Pfizer Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- GSK plc (U.K.)
- Sanofi (France)
- Bayer AG (Germany)
- Bristol-Myers Squibb Company (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Eli Lilly and Company (U.S.)
- Amgen Inc. (U.S.)
- Guardant Health, Inc. (U.S.)
- Natera, Inc. (U.S.)
What are the Recent Developments in Global Cowden’s Disease Treatment Market?
- In March 2025, an international multicenter cohort study published in npj Precision Oncology reported on 701 individuals with germline PTEN variants, identifying a broader range of malignancies associated with Cowden’s disease beyond the traditional breast, thyroid, and kidney cancers. The discovery of “non-component cancers” such as sarcomas and prostate cancer broadens the disease’s oncological profile
- In March 2025, researchers reported that commonly used anticancer drugs targeting the PI3K/AKT/mTOR pathway specifically rapamycin and capivasertib significantly reduced vascular malformations and lesion-associated pain in preclinical models and two early-stage patients with PHTS, suggesting a potential repurposing opportunity for these agents in Cowden’s syndrome management
- In August 2024, Cureus Journal of Medical Science published a case series detailing patients with Cowden’s syndrome presenting with varied cutaneous lesions and systemic manifestations, including thyroid carcinoma in a PTEN-mutated case. The report emphasized the diagnostic complexity and multidisciplinary management required for these patients
- In July 2023, the Blue Cross Blue Shield Association’s Federal Employee Program (FEP) updated its Medical Policy on Genetic Testing for PTEN Hamartoma Tumor Syndrome (PHTS). The revised policy enhanced coverage clarity and clinical utility guidelines for PTEN testing in individuals suspected of having Cowden’s disease
- In August 2022, a Phase II clinical trial investigating the AKT inhibitor TAS-117 demonstrated activity in patients with PTEN-inactivated advanced solid tumors, including those carrying germline PTEN mutations associated with Cowden’s disease. Conducted across multiple international sites, the study showcased the therapeutic potential of targeting the PI3K/AKT pathway in PTEN-deficient malignancies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.