Global Crispr Edited Stem Cell Therapy Market
Market Size in USD Million
CAGR :
% 
 USD
120.22 Million
USD
560.49 Million
2024
2032
USD
120.22 Million
USD
560.49 Million
2024
2032
| 2025 –2032 | |
| USD 120.22 Million | |
| USD 560.49 Million | |
|
|
|
|
CRISPR-Edited Stem Cell Therapy Market Size
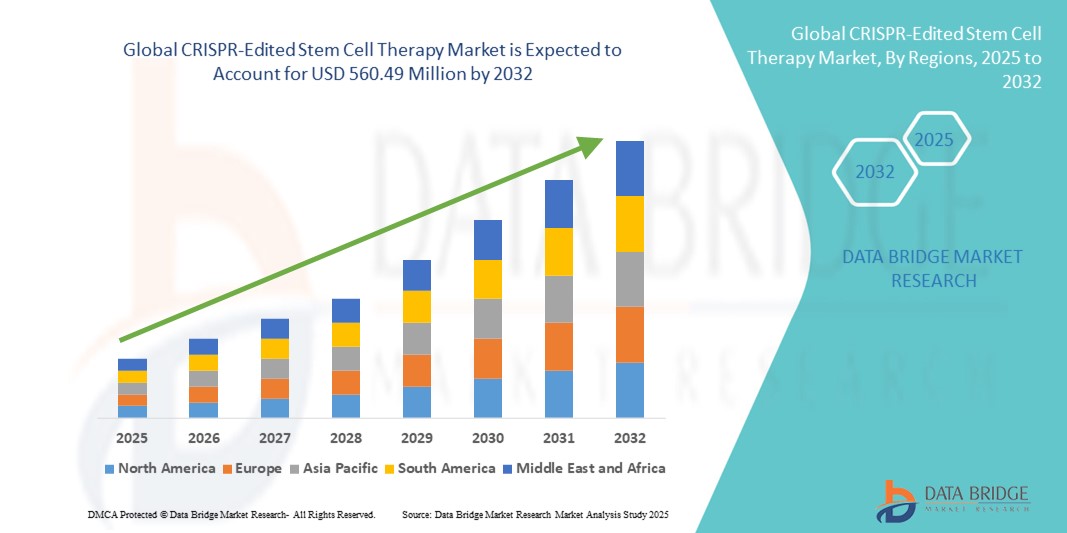
- The global CRISPR-Edited Stem Cell Therapy market size was valued at USD 120.22 million in 2024 and is expected to reach USD 560.49 million by 2032, at a CAGR of 21.22% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress within gene editing technologies and stem cell research, leading to increased digitalization and precision in therapeutic development across both academic and clinical settings
- Furthermore, rising demand for personalized medicine, targeted therapies, and next-generation treatments is establishing CRISPR-edited stem cell therapy as a transformative solution of choice. These converging factors are accelerating the uptake of CRISPR-Edited Stem Cell Therapy solutions, thereby significantly boosting the industry's growth
CRISPR-Edited Stem Cell Therapy Market Analysis
- CRISPR-Edited Stem Cell Therapy, a cutting-edge medical technology combining gene-editing precision with regenerative potential, is emerging as a transformative solution for treating genetic disorders, degenerative diseases, and rare conditions by editing patient-derived stem cells before reinfusion
- The rapidly increasing demand for CRISPR-edited therapies is primarily driven by the growing prevalence of inherited and chronic diseases, rising investment in advanced genetic research, and the expanding capabilities of CRISPR-Cas9 systems in clinical applications
- North America dominated the CRISPR-Edited Stem Cell Therapy market with the largest revenue share of 42.6% in 2024, supported by the early adoption of gene therapies, strong funding in biotech innovation, a robust pipeline of clinical trials, and a favorable regulatory environment. The U.S. has emerged as a global hub for stem cell therapy development, with institutions such as the NIH and major biopharma companies driving commercialization
- Asia-Pacific is projected to be the fastest-growing region in the CRISPR-Edited Stem Cell Therapy market with a CAGR of 24.7% from 2025 to 2032, attributed to a rising burden of genetic diseases, supportive government policies for biotech innovation, and increased research collaborations in countries like China, Japan, and South Korea
- The CRISPR/Cas9 segment dominated the CRISPR-Edited Stem Cell Therapy market with a market share of 67.4% in 2024, owing to its well-established precision, ease of use, and broad applicability in both research and clinical settings. Its proven success in gene editing has positioned CRISPR/Cas9 as the preferred choice among scientists and developers for engineering stem cells across various therapeutic applications
Report Scope and CRISPR-Edited Stem Cell Therapy Market Segmentation
|
Attributes |
CRISPR-Edited Stem Cell Therapy Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
CRISPR-Edited Stem Cell Therapy Market Trends
“Advancing Therapeutic Precision and Disease Targeting”
- A significant and accelerating trend in the global CRISPR-Edited Stem Cell Therapy market is the increasing focus on enhancing therapeutic precision and expanding the spectrum of treatable diseases, particularly those with a genetic basis. The fusion of gene editing tools with stem cell platforms is transforming regenerative medicine by enabling highly specific gene corrections that were previously unattainable
- For instance, CRISPR/Cas9-modified hematopoietic stem cells are being used to treat blood disorders such as sickle cell anemia and beta-thalassemia, showing high efficacy and durability in early clinical trials. Similarly, autologous CRISPR-edited neural stem cells are under investigation for targeted treatment of neurological disorders like Parkinson’s disease and ALS, offering personalized solutions for complex conditions
- This integration allows for permanent correction of pathogenic mutations, reducing or even eliminating the need for chronic treatment. Moreover, the use of stem cells as delivery vehicles for corrected genes or functional cells is unlocking new potential in oncology, ophthalmology, and cardiovascular repair, expanding the therapeutic reach of gene editing technologies
- The convergence of stem cell therapy with advanced gene editing is enabling in vivo and ex vivo treatment models that are more adaptable, scalable, and clinically impactful. Leading players are developing novel delivery platforms such as lipid nanoparticles and viral vectors to facilitate safer and more efficient gene insertion or deletion
- This trend towards precision medicine and disease modification is fundamentally reshaping the expectations of both healthcare providers and patients. Consequently, companies and research institutes are investing heavily in platform technologies, manufacturing scalability, and regulatory alignment to bring next-generation therapies to market faster and more effectively
- The demand for CRISPR-edited stem cell therapies that offer high specificity, long-term efficacy, and potential cures is growing rapidly across multiple therapeutic areas, as patients and providers seek innovative solutions for genetic and chronic disorders that have long lacked effective treatments
CRISPR-Edited Stem Cell Therapy Market Dynamics
Driver
“Growing Need Due to Rising Demand for Targeted and Regenerative Therapies”
- The increasing prevalence of genetic disorders, cancers, and chronic diseases, coupled with the rising demand for personalized and regenerative treatment approaches, is significantly driving the growth of the CRISPR-Edited Stem Cell Therapy market
- For instance, in April 2024, Vertex Pharmaceuticals and CRISPR Therapeutics received regulatory fast-track designation for their CRISPR-Cas9 edited therapy targeting beta-thalassemia and sickle cell disease, reflecting strong commercial and clinical momentum in the sector
- As researchers and clinicians seek more precise, long-lasting treatments, CRISPR-edited stem cells offer transformative capabilities in repairing or replacing damaged tissues at the genetic level, surpassing conventional therapies in potential efficacy and durability
- Furthermore, the growing body of supportive clinical trial data and technological advancements in gene editing platforms like CRISPR/Cas9 are encouraging increased investment and collaboration across pharmaceutical and biotech sectors
- The promise of one-time, curative interventions through ex vivo and in vivo gene-editing is fueling the adoption of CRISPR-Edited Stem Cell Therapies in oncology, hematology, and rare disease segments. Increasing public awareness and favorable regulatory pathways in key regions such as the U.S. and Europe are further supporting the market’s rapid expansion
Restraint/Challenge
“Regulatory Uncertainty and High Development Costs”
- The complex ethical, safety, and regulatory landscape surrounding gene editing poses a significant challenge for market participants. The long-term risks of genome alterations, especially germline editing, continue to provoke scrutiny and debate
- For instance, despite promising early results, many CRISPR-edited stem cell therapies remain in investigational stages due to the extensive preclinical and clinical validation required for approval
- Addressing these regulatory hurdles through transparent data reporting, rigorous safety assessments, and global harmonization of gene therapy guidelines is critical for sustained progress. Leading companies such as Editas Medicine and Intellia Therapeutics have emphasized their compliance-driven development frameworks to build stakeholder confidence
In addition, the high cost of CRISPR-editing platforms, delivery systems, and stem cell manipulation technologies can be prohibitive, particularly for small firms or in lower-income healthcare settings. The need for specialized infrastructure and trained personnel also elevates the entry barrier - While costs are expected to decline with scale and technology maturation, affordability remains a key concern. Overcoming these challenges through public-private partnerships, government support, and broader access to clinical trials will be vital for unlocking the full potential of the CRISPR-Edited Stem Cell Therapy market
CRISPR-Edited Stem Cell Therapy Market Scope
The market is segmented on the basis of type, editing technique, delivery method, and application.
• By Type
On the basis of type, the CRISPR-edited stem cell therapy market is segmented into allogeneic stem cell therapy and autologous stem cell therapy. The allogeneic stem cell therapy segment dominated the largest market revenue share of 58.3% in 2024, driven by its application in treating various hematological and genetic disorders where matched donors are available. Its scalability and potential for off-the-shelf treatments make it favorable for large patient populations.
The autologous stem cell therapy segment is expected to witness the fastest growth rate of 22.6% from 2025 to 2032, as personalized medicine gains momentum. These therapies, derived from a patient’s own cells, reduce the risk of immune rejection and are increasingly being investigated in oncology and rare disease applications.
• By Editing Technique
On the basis of editing technique, the CRISPR-edited stem cell therapy market is segmented into CRISPR/Cas9, CRISPR/Cas12, CRISPR/Cas13, and Others. CRISPR/Cas9 held the largest market share of 67.4% in 2024 due to its well-established precision, ease of use, and broad research and clinical applicability.
The CRISPR/Cas12 segment is projected to register the fastest CAGR of 24.1% during the forecast period, as it offers a more compact structure and increased target flexibility, making it highly suitable for diagnostics and therapeutic applications.
• By Delivery Method
On the basis of delivery method, the CRISPR-edited stem cell therapy market is segmented into Ex Vivo Gene Editing and In Vivo Gene Editing. The Ex Vivo Gene Editing segment accounted for the largest revenue share of 62.9% in 2024, due to its controlled laboratory environment which allows greater precision and safety before reinfusing edited cells into the patient.
The In Vivo Gene Editing segment is expected to expand at the highest CAGR of 25.7% from 2025 to 2032, fueled by advancements in delivery systems like viral vectors and lipid nanoparticles, enabling direct gene editing within the patient’s body for conditions like retinal disorders and liver-based genetic diseases.
• By Application
On the basis of application, the CRISPR-Edited Stem Cell Therapy market is segmented into oncology, blood disorders, neurological disorders, ophthalmology, and others. Oncology accounted for the largest market share of 39.2% in 2024, driven by strong clinical research in using edited immune and stem cells to target various cancers, including leukemia and solid tumors.
The neurological disorders segment is anticipated to grow at the fastest CAGR of 26.4%, owing to rising incidences of conditions like Parkinson’s and Huntington’s disease, and the potential of CRISPR-edited stem cells to repair or replace damaged neurons.
CRISPR-Edited Stem Cell Therapy Market Regional Analysis
- North America dominated the CRISPR-edited stem cell therapy market with the largest revenue share of 42.6% in 2024, driven by robust investments in gene editing research, an advanced healthcare infrastructure, and supportive regulatory frameworks for clinical trials involving regenerative medicine
- Consumers in the region benefit from high awareness, favorable reimbursement policies, and accelerated clinical trial approvals. This creates a strong demand for CRISPR-edited stem cell therapies, especially for conditions such as cancer, blood disorders, and inherited diseases
- The U.S. leads the growth due to a high concentration of biotech companies, extensive NIH funding, and the availability of skilled genomic researchers, strengthening its leadership in the global market
U.S. CRISPR-Edited Stem Cell Therapy Market Insight
The U.S. CRISPR-edited stem cell therapy market accounted for 81.3% of the North American CRISPR-edited stem cell therapy market in 2024, driven by strong institutional support, advanced R&D capabilities, and a growing pipeline of CRISPR-related clinical trials. Rapid advancements in personalized medicine and gene therapy, increased venture capital investments, and FDA designations for orphan and breakthrough therapies are major factors contributing to market expansion in the U.S.
Europe CRISPR-Edited Stem Cell Therapy Market Insight
The Europe CRISPR-edited stem cell therapy market held a revenue share of 29.5% in the global CRISPR-edited stem cell therapy market in 2024, driven by stringent but evolving regulatory frameworks, academic research excellence, and national investments in genomics and regenerative medicine. The region is witnessing a rise in early-phase trials for genetic and neurological disorders, with countries like Germany, the U.K., and France leading innovation and commercial adoption.
U.K. CRISPR-Edited Stem Cell Therapy Market Insight
The U.K. CRISPR-edited stem cell therapy market contributed 26.7% of the European CRISPR-edited stem cell therapy market revenue in 2024, supported by NHS genomic integration, the 100,000 Genomes Project, and government funding through initiatives like the Biomedical Catalyst.
Germany CRISPR-Edited Stem Cell Therapy Market Insight
The Germany CRISPR-edited stem cell therapy market held 24.2% of the European market share in 2024, driven by a strong clinical research ecosystem, early adoption of ATMPs, and emphasis on ethical practices in gene editing. Germany’s focus on clinical trials in oncology and rare diseases is further fueling market growth.
Asia-Pacific CRISPR-Edited Stem Cell Therapy Market Insight
The Asia-Pacific CRISPR-edited stem cell therapy market is projected to grow at the fastest CAGR of 24.7% from 2025 to 2032, and it held a revenue share of 18.7% in 2024, driven by increased government spending on precision medicine and expanding biotech manufacturing hubs in China, Japan, and India. Rising demand for advanced therapies, growing awareness, and regulatory reforms are accelerating the deployment of CRISPR-based therapies across the region.
Japan CRISPR-Edited Stem Cell Therapy Market Insight
The Japan CRISPR-edited stem cell therapy market accounted for 27.6% of the Asia-Pacific CRISPR-edited stem cell therapy market in 2024, driven by expedited regulatory pathways, the country’s aging population, and its leadership in stem cell innovation through institutions like RIKEN and CiRA.
China CRISPR-Edited Stem Cell Therapy Market Insight
The China CRISPR-edited stem cell therapy market held the largest share in Asia-Pacific at 38.4% in 2024, attributed to strong government support for biotechnology, rising investments in clinical trials, and the country's ambition to become a global genomics leader. China’s push for local innovation and fast-paced development of CRISPR technologies for oncology and inherited diseases is significantly boosting growth.
CRISPR-Edited Stem Cell Therapy Market Share
The CRISPR-Edited stem cell therapy industry is primarily led by well-established companies, including:
- CRISPR Therapeutics AG (Switzerland)
- Editas Medicine, Inc. (U.S.)
- Intellia Therapeutics, Inc. (U.S.)
- Sangamo Therapeutics, Inc. (U.S.)
- Caribou Biosciences, Inc. (U.S.)
- Cellectis S.A. (France)
- Thermo Fisher Scientific Inc. (U.S.)
- Takara Bio Inc. (Japan)
- Merck KGaA (Germany)
- Horizon Discovery Ltd. (U.K.)
- Synthego Corporation (U.S.)
- Beam Therapeutics Inc. (U.S.)
- Precision BioSciences, Inc. (U.S.)
- Novartis AG (Switzerland)
- AstraZeneca (U.K.)
- EditForce Inc. (Japan)
- Oxford Biomedica (U.K.)
- BlueRock Therapeutics (U.S.)
- GenScript Biotech Corporation (China)
- Regenxbio Inc. (U.S.)
- Pluristem Therapeutics Inc. (Israel)
- Verve Therapeutics (U.S.)
- Graphite Bio, Inc. (U.S.)
- Cellink AB (Sweden)
Latest Developments in Global CRISPR-Edited Stem Cell Therapy Market
- In December 2023, the U.S. Food and Drug Administration (FDA) approved Casgevy (exagamglogene autotemcel), developed by Vertex Pharmaceuticals and CRISPR Therapeutics. It became the world’s first CRISPR-based gene therapy approved to treat sickle cell disease (SCD) and transfusion-dependent beta-thalassemia (TDT). This groundbreaking approval marked a historic milestone in the CRISPR-edited stem cell therapy landscape
- In April 2024, Cellistic, a Belgian biotechnology firm, announced the acquisition of Artisan Bio’s STAR-CRISPR Cas12 platform. This strategic move allows Cellistic to offer more precise gene-editing tools for the development of induced pluripotent stem cell (iPSC)-derived allogeneic therapies, signaling growing commercial interest in CRISPR platforms
- In April 2025, researchers published new findings demonstrating a dual-tropic CRISPR-Cas9-based strategy that edits hematopoietic stem/progenitor cells to confer resistance against both R5 and X4 strains of HIV. This approach is seen as a promising potential treatment for long-term viral control in HIV-positive individuals
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.