Global Dengue Vaccine Market
Market Size in USD Million
CAGR :
% 
 USD
661.27 Million
USD
1,602.53 Million
2024
2032
USD
661.27 Million
USD
1,602.53 Million
2024
2032
| 2025 –2032 | |
| USD 661.27 Million | |
| USD 1,602.53 Million | |
|
|
|
|
Dengue Vaccine Market Size
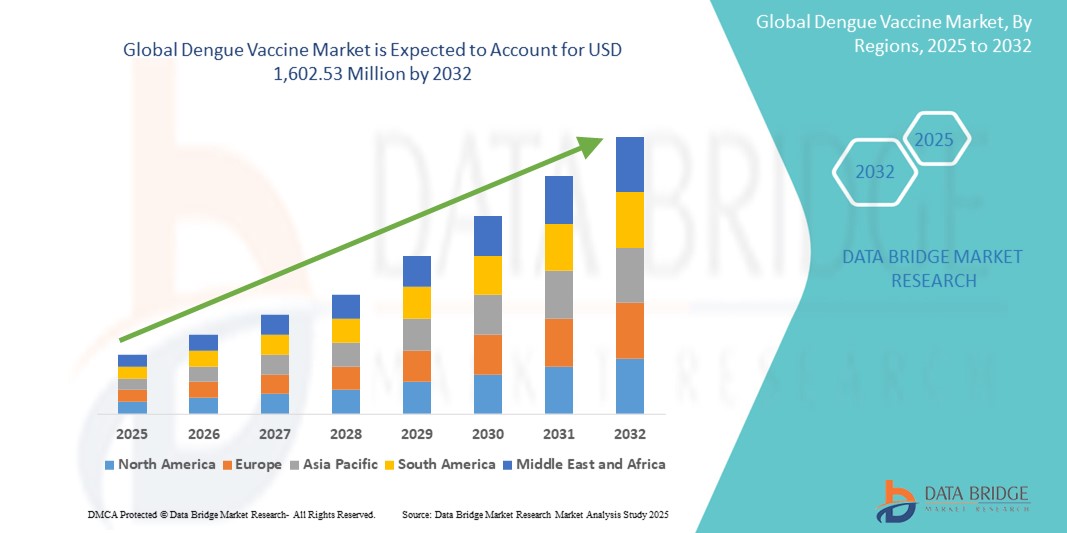
- The global dengue vaccine market size was valued at USD 661.27 million in 2024 and is expected to reach USD 1,602.53 million by 2032, at a CAGR of 11.70% during the forecast period
- The market growth is largely fueled by the rising global prevalence of dengue fever, increasing public health awareness, and government-led immunization initiatives aimed at combating mosquito-borne diseases, particularly in endemic regions such as Southeast Asia, Latin America, and parts of Africa
- Furthermore, the growing demand for safe, effective, and long-term immunization solutions is establishing dengue vaccines as a critical component of public health strategies. These converging factors are accelerating the uptake of dengue vaccine solutions, thereby significantly boosting the dengue vaccine market's growth
Dengue Vaccine Market Analysis
- Dengue vaccines, designed to protect against the dengue virus transmitted by mosquitoes, are gaining traction as a critical public health tool due to the increasing incidence of dengue infections in tropical and subtropical regions. The growing global burden of dengue fever, coupled with efforts to reduce morbidity and mortality, is driving demand for effective and accessible dengue vaccination programs
- The rising awareness about the health impacts of dengue, combined with supportive government immunization initiatives and growing healthcare infrastructure in endemic regions, has significantly fueled market growth
- North America dominated the dengue vaccine market with the largest revenue share of 46.0% in 2024, driven by strong R&D investment, early regulatory approvals, and growing traveler immunization requirements. The U.S. particularly experienced substantial market expansion owing to the introduction of dengue vaccines for travelers and military personnel deployed to dengue-endemic regions
- Asia-Pacific is expected to be the fastest growing region in the dengue vaccine market during the forecast period, with a projected CAGR of 23.6%. This growth is attributed to the high prevalence of dengue cases in countries such as India, Indonesia, the Philippines, and Thailand, alongside rising government support for mass immunization campaigns and improved vaccine accessibility
- The parenteral segment dominated the dengue vaccine market with a market share of 67.5% in 2024, as most dengue vaccines currently available are administered through injection and require medical supervision. This method ensures accurate dosing and effectiveness, particularly in immunization programs conducted by healthcare institutions across endemic regions
Report Scope and Dengue Vaccine Market Segmentation
|
Attributes |
Dengue Vaccine Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Dengue Vaccine Market Trends
“Growing Public Health Awareness and Preventive Healthcare Initiatives”
- A significant and accelerating trend in the global dengue vaccine market is the increasing public awareness surrounding mosquito-borne diseases and the importance of vaccination as a preventive measure. This rising health consciousness, particularly in endemic regions, is fueling demand for effective and long-lasting dengue vaccines
- For instance, government-backed campaigns and global health organization initiatives such as those by the World Health Organization (WHO) and GAVI, the Vaccine Alliance, are helping to improve vaccine coverage in high-risk regions including Southeast Asia, Latin America, and Africa. These efforts are significantly enhancing public understanding of the benefits of dengue vaccination
- Vaccine manufacturers are increasingly focusing on developing multivalent formulations capable of targeting all four dengue virus serotypes. The success of Takeda’s QDENGA vaccine in gaining regulatory approvals in countries like Indonesia and Brazil reflects this shift toward more comprehensive immunization solutions
- The increasing involvement of both public and private healthcare sectors in rolling out dengue vaccination programs has also led to improvements in cold chain infrastructure, vaccine storage logistics, and last-mile delivery—critical factors for ensuring vaccine efficacy in rural and urban populations
- In addition, the emergence of travel vaccination markets—driven by the rise in international travel to dengue-endemic areas—is creating a niche demand for pre-travel dengue immunization. Clinics and pharmacies offering travel-related vaccination services are expanding their offerings to include dengue vaccines, especially in North America and Europe
- This trend toward preventive healthcare and population-wide immunization is fundamentally reshaping global strategies for combating vector-borne diseases. As countries increasingly invest in dengue control measures, the demand for safe and effective vaccines is expected to grow substantially, offering long-term opportunities for pharmaceutical developers and public health authorities
Dengue Vaccine Market Dynamics
Driver
“Growing Need Due to Rising Global Dengue Burden and Vaccine Rollouts”
- The increasing incidence of dengue fever, especially in tropical and subtropical regions, coupled with climate change and urbanization, is significantly driving the demand for dengue vaccines. Governments and global health organizations are accelerating immunization programs to prevent outbreaks and reduce the healthcare burden
- For instance, in May 2024, Takeda’s dengue vaccine QDENGA was added to the expanded immunization schedules in Brazil and Indonesia following promising clinical trial results. Such strategies by key companies are expected to drive the Dengue Vaccine industry growth in the forecast period
- As awareness about dengue prevention grows and access to vaccination expands, more individuals are opting for immunization against the disease. The convenience of preventive vaccination, especially in high-risk populations such as travelers, children, and healthcare workers, is further enhancing market adoption
- Furthermore, collaborations between international bodies like WHO and Gavi, and the inclusion of dengue vaccines in national immunization programs, are making these vaccines more accessible, particularly in low- and middle-income countries
- The rise in vector-borne disease surveillance, support for endemic country initiatives, and the ongoing development of next-generation vaccines targeting multiple serotypes contribute to long-term market growth
Restraint/Challenge
“Concerns Regarding Vaccine Safety and High Development Costs”
- Concerns over vaccine safety, particularly in individuals with no prior dengue exposure, pose a significant challenge to wider market acceptance. Previous controversies, such as with Sanofi’s Dengvaxia in the Philippines, have made both healthcare providers and the public cautious
- For instance, WHO continues to emphasize the need for serostatus testing before Dengvaxia administration due to risks in seronegative individuals, which limits large-scale use and complicates rollout logistics
- Addressing these concerns through improved diagnostic tools, real-world safety data, and transparent communication is critical to rebuilding public trust. Newer vaccines like QDENGA are positioned as safer alternatives with fewer restrictions
In addition, the high cost of vaccine development, manufacturing, and distribution—especially for multivalent vaccines—can be a barrier to adoption, particularly in low-resource settings - While global support through funding agencies and partnerships is helping to bridge access gaps, consistent investment, regulatory streamlining, and education efforts are vital for overcoming these challenges and achieving broad vaccine coverage in endemic regions
Dengue Vaccine Market Scope
The market is segmented on the basis of type, treatment, route of administration, end-users, and distribution channel.
• By Type
On the basis of type, the dengue vaccine market is segmented into live attenuated vaccine, chimeric live attenuated vaccine, inactivated vaccine, subunit vaccine, and nucleic acid based vaccine. The live attenuated vaccine segment dominated the largest market revenue share of 42.3% in 2024, owing to its broad acceptance and usage in endemic regions.
The nucleic acid based vaccine segment is expected to witness the fastest CAGR of 22.3% from 2025 to 2032, due to the growing adoption of mRNA and DNA platforms offering rapid development and scalability.
• By Treatment
On the basis of treatment, the dengue vaccine market is segmented into diuretic, anti-allergic, blood thinners, and others. The anti-allergic segment accounted for the largest market share of 38.7% in 2024, driven by its critical role in symptom management of rashes and allergic reactions.
The blood thinners segment is projected to grow at the fastest CAGR of 18.6% from 2025 to 2032, due to rising focus on severe dengue complications involving vascular and clotting disorders.
• By Route of Administration
On the basis of route of administration, the dengue vaccine market is segmented into oral, parenteral, and others. The parenteral segment held the highest revenue share of 67.5% in 2024, as most current dengue vaccines are injectable and administered under medical supervision.
The oral segment is expected to grow at the fastest CAGR of 19.2% from 2025 to 2032, driven by innovation in oral delivery methods, especially for pediatric use and mass immunization.
• By End-Users
On the basis of end-users, the dengue vaccine market is segmented into hospitals, homecare, specialty clinics, and others. The hospitals segment led the market with a revenue share of 49.3% in 2024, owing to their critical role in vaccine delivery, dengue patient care, and storage facilities.
The specialty clinics segment is anticipated to grow at the fastest CAGR of 17.4% from 2025 to 2032, due to increasing preference for outpatient vaccination services in urban areas.
• By Distribution Channel
On the basis of distribution channel, the dengue vaccine market is segmented into hospital pharmacy, online pharmacy, and retail pharmacy. The hospital pharmacy segment dominated with a market share of 45.8% in 2024, supported by robust distribution networks linked to healthcare institutions.
The online pharmacy segment is projected to expand at the fastest CAGR of 20.1% from 2025 to 2032, fueled by growing e-health platforms and doorstep vaccine delivery services.
Dengue Vaccine Market Regional Analysis
- North America dominated the dengue vaccine market with the largest revenue share of 46.0% in 2024, driven by rising awareness of dengue outbreaks, proactive immunization strategies, and strong governmental support. The region benefits from advanced healthcare infrastructure and early approval of vaccines, such as TAK-003 (Qdenga) and Dengvaxia, facilitating swift distribution and uptake across the U.S., Mexico, and Caribbean nations
- The increasing prevalence of dengue cases, particularly in warmer U.S. states and travel-related regions, has fueled demand for preventive vaccination. Public health campaigns and collaborations with pharmaceutical companies have also contributed to regional dominance
- This robust market expansion is further supported by high healthcare spending, rapid adoption of innovative vaccine technologies, and heightened traveler immunization programs targeting endemic zones
U.S. Dengue Vaccine Market Insight
The U.S. dengue vaccine market captured the largest revenue share of 71% within North America in 2024, propelled by ongoing R&D investments, FDA-regulated approval processes, and growing concerns over vector-borne diseases due to climate change. The market is further driven by the country’s strong biopharmaceutical presence and high demand for traveler vaccines, particularly among military personnel and tourists. Moreover, the U.S. continues to see heightened interest in clinical trials and public-private collaborations aimed at strengthening vaccine pipelines.
Europe Dengue Vaccine Market Insight
The Europe dengue vaccine market is projected to expand at a substantial CAGR during the forecast period, fueled by increased awareness of dengue prevention, especially among travelers, migrants, and populations in overseas territories. The region’s stringent healthcare policies, travel medicine initiatives, and focus on tropical disease management are driving vaccine demand. Countries such as France, Germany, and the U.K. are key contributors, with expanding participation in international dengue vaccine research programs.
U.K. Dengue Vaccine Market Insight
The U.K. dengue vaccine market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by a robust global travel culture and rising demand for pre-travel vaccinations. Public health awareness campaigns, along with NHS travel vaccination programs, are increasing the uptake of dengue vaccines. The U.K. also maintains active involvement in global health partnerships that promote access to vaccines in endemic regions, indirectly supporting local growth through manufacturing and export partnerships.
Germany Dengue Vaccine Market Insight
The Germany dengue vaccine market is expected to expand at a considerable CAGR during the forecast period, driven by strong participation in international vaccine development, government-backed health education programs, and high healthcare expenditure. Germany’s emphasis on digital health and innovation also facilitates effective tracking of dengue cases, improving risk-based vaccine deployment in both domestic and overseas populations.
Asia-Pacific Dengue Vaccine Market Insight
The Asia-Pacific dengue vaccine market is poised to grow at the fastest CAGR of 23.6% from 2025 to 2032. The region sees the highest dengue burden globally, especially in countries like India, Indonesia, Thailand, and the Philippines. Government-supported mass vaccination campaigns, WHO-led awareness initiatives, and rapid urbanization are key contributors to market growth. The presence of domestic vaccine manufacturers and expanding access in rural and semi-urban areas are boosting uptake.
Japan Dengue Vaccine Market Insight
The Japan dengue vaccine market is gaining momentum, growing at a projected notable CAGR during the forecast period. Demand is driven by rising travel to endemic countries and dengue cases reported in returning travelers. Japan’s precision health approach, coupled with public and private investment in tropical disease prevention, supports growing adoption. Government efforts to stockpile vaccines for future outbreaks and strong diagnostic capabilities also contribute to market expansion.
China Dengue Vaccine Market Insight
The China dengue vaccine market accounted for the largest market revenue share of 44.9% in Asia-Pacific in 2024, driven by a rising number of dengue cases in southern provinces, expanding public vaccination programs, and robust domestic production. The country’s focus on tropical disease control, coupled with heavy investment in biotech manufacturing and vaccine R&D, is fueling rapid growth. Governmental emphasis on health security and emergency preparedness ensures the steady integration of dengue vaccines into broader public health frameworks.
Dengue Vaccine Market Share
The dengue vaccine industry is primarily led by well-established companies, including:
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sanofi (France)
- Novartis AG (Switzerland)
- GSK plc (U.K.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Takeda Pharmaceutical Company Limited (Japan)
- BIO-MED (India)
- Intercept Pharmaceuticals, Inc. (U.K.)
- Emcure Pharmaceuticals Ltd (India)
- Changchun BCHT Biotechnology Co. (China)
- Novo Medi Sciences Pvt. Ltd. (India)
Latest Developments in Global Dengue Vaccine Market
- In February 2024, Takeda and Biological E announced a collaboration to produce 50 million dengue vaccines, enhancing efforts to combat the disease. In addition, Miltenyi Biotec unveiled the Hyderabad Center for Cell and Gene Therapy Services, focusing on advanced therapeutic solutions. The BioAsia 2024 event showcased significant breakthroughs in the pharmaceutical industry, highlighting innovations and advancements in healthcare
- In April 2023, health authorities in Argentina approved the use of a Japanese dengue vaccine, which requires administration in two doses spaced three months apart. This decision marks a significant step in enhancing dengue prevention efforts within the country. The two-dose regimen is designed to ensure optimal immunity against the disease
- In May 2023, Takeda announced that its dengue vaccine, QDENGA, received multiple approvals, including one from Brazil's National Health Surveillance Agency in March 2023. This approval permits the vaccine's use in individuals aged 4 to 60 years, offering protection against all four dengue virus serotypes. Takeda's advancements in securing these approvals demonstrate its ongoing efforts to combat dengue fever effectively
- In March 2021, Takeda Pharmaceutical Company Limited announced that its dengue vaccine candidate, TAK-003, received approval from the European Medicines Agency (EMA) for use in preventing outbreaks among individuals aged 4 to 60. The company plans to seek regulatory approvals for this vaccine in several countries, including Argentina, Brazil, Colombia, Indonesia, Malaysia, Mexico, Singapore, Sri Lanka, and Thailand throughout 2021. This move reflects Takeda's commitment to expanding access to dengue prevention globally
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.