Global Dermatitis Market
Market Size in USD Billion
CAGR :
% 
 USD
5.92 Billion
USD
11.04 Billion
2021
2029
USD
5.92 Billion
USD
11.04 Billion
2021
2029
| 2022 –2029 | |
| USD 5.92 Billion | |
| USD 11.04 Billion | |
|
|
|
|
Market Analysis and Size
In recent years, the dermatitis market is anticipated to grow rapidly during the forecast period. Atopic dermatitis affects roughly 31.6 million people in the United States as per the allergy asthma network 2020. Atopic dermatitis affects 15-20 percent of children and 1 to 3 percent of adults over the world. Additionally, continuously changing lifestyle results in the surging use of cosmetics, face creams, sunscreen and fluorinated toothpaste. Along with this, the prevalence rate of lifestyle or chronic diseases is high, including the growing use of topical and inhaled steroids. These are the causative factors increasing the risk of dermatitis. Hence this led to the high demand for dermatitis in the market.
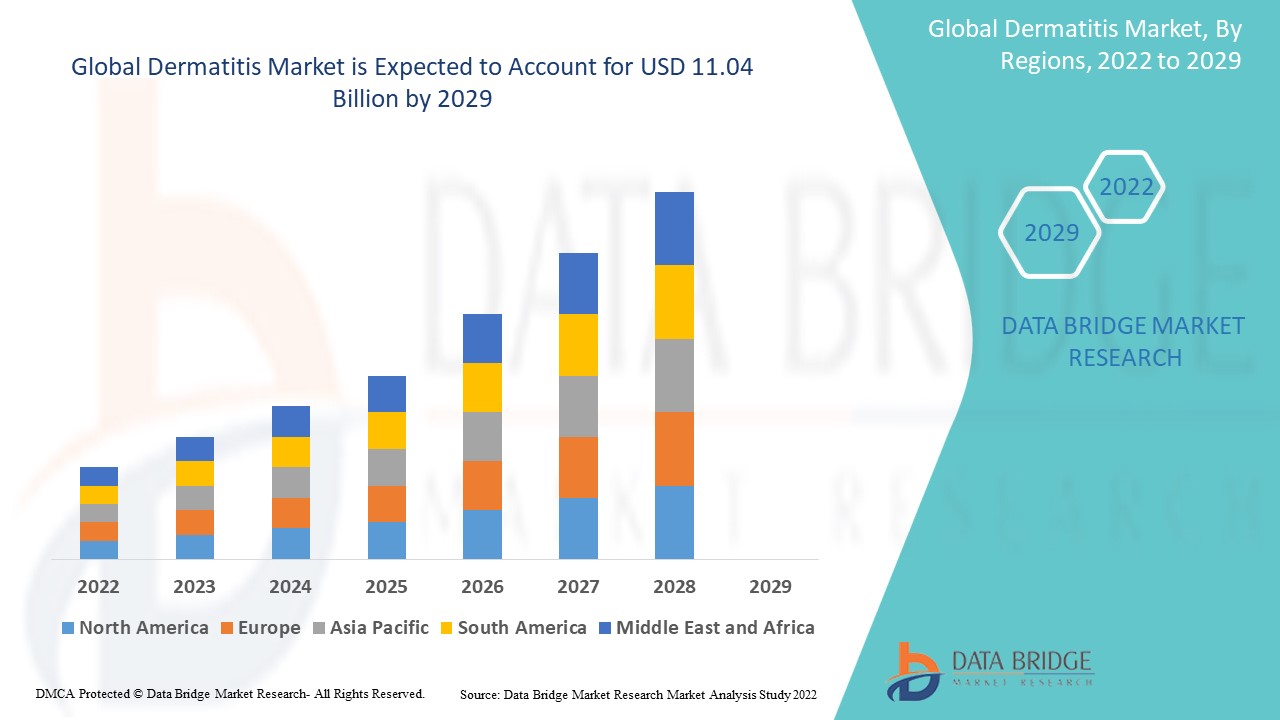
Data Bridge Market Research analyses that the dermatitis market was valued at USD 5.92 billion in 2021 and is expected to reach USD 11.04 billion by 2029, registering a CAGR of 8.10% during the forecast period of 2022 to 2029. The market report curated by the Data Bridge Market Research team includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Market Definition
Dermatitis is a term that encompasses a variety of illnesses, including skin inflammation. Most people's early stages of dermatitis are marked by red, dry, and itchy skin. More severe dermatitis might cause crusty scales, painful cracks, or fluid-filled blisters. Because numerous items can irritate the skin, a doctor will strive to narrow down the diagnosis to a specific type of dermatitis, even though most types of skin irritation and inflammation have comparable treatments.
Report Scope and Market Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Contact Dermatitis, Atopic Dermatitis, Diaper Dermatitis, Nummular Dermatitis, Dyshidrotic Dermatitis, Perioral Dermatitis, Neurodermatitis, Seborrheic Dermatitis, Stasis Dermatitis), Treatment (Antibiotics, Antihistamines, Calcineurin Inhibitors, Topical Corticosteroids, Emollients, Topical Antiseptic, Others), Diagnosis (Blood Tests, Allergy Skin Test, Skin Biopsy), Route of Administration (Oral, Topical, Others), Dosage Form (Tablets, Ointments, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (US), Teva Pharmaceutical Industries Ltd.(Israel), Sanofi (France), Pfizer Inc. (US), GlaxoSmithKline plc (UK), Novartis AG (Switzerland), AstraZeneca (UK), Johnson & Johnson (US), Bayer AG (Germany), Sun Pharmaceutical Industries Ltd. (India), LEO Pharma A/S (Denmark), Aurobindo Pharma (India), Lupin (India), Eli Lilly and Company (US), Boehringer Ingelheim International GmbH. (Germany), Bausch Health Companies Inc. (Canada), Bristol-Myers Squibb Company (US), AbbVie Inc. (US), Allergan (Ireland) |
|
Market Opportunities |
|
Dermatitis Market Dynamics
Drivers
- Surging prevalence rate of allergic reactions
The rising prevalence of allergic reactions, with food allergies playing a prominent role is estimated to enhance the dermatitis market's growth. According to a 2020 report from the Food Allergy Research and Education, 32 million Americans have food allergies, including 5.6 million children under the age of 18. Approximately 40% of children with food allergies are allergic to many foods.
- Increasing investment for healthcare infrastructure
Another significant factor influencing the growth rate of dermatitis market is the rising healthcare expenditure which helps in improving its infrastructure. Also, various government organizations aims to improve the healthcare infrastructure by increasing funding and this will further influence the market dynamics.
- Growing number of geriatric population
The surging geriatric population is estimated to enhance the market's expansion during the forecast period of 2022-2029. According to the World Health Organization (WHO), the worldwide geriatric population, which was estimated to be over 524 million in 2010, is expected to increase to nearly 2 billion by 2050. Due to their compromised immune systems, geriatrics are more susceptible to get chronic ailments, further estimated to enhance the market's growth rate.
Furthermore, rising initiatives by public and private organizations to spread awareness will expand the dermatitis market. Additionally, increasing environmental pollution and rising incidences of hormonal imbalances will expand the dermatitis market.
Opportunities
- Increase in the number of research and development activities
Moreover, the market's growth is fueled by an increase in the number of research and development activities. This will provide beneficial opportunities for the dermatitis market growth. Along with this, rising drug approvals and launches will further propel the market's growth rate.
Moreover, rising investment for the development of advanced technologies and an increase in the number of emerging markets will provide beneficial opportunities for the dermatitis market growth during the forecast period.
Restraints/Challenges
On the other hand, high cost high cost associated with the treatment will obstruct the growth rate of market. The dearth of skilled professionals and lack of healthcare infrastructure in developing economies will challenge the dermatitis market. Additionally, strict regulatory policies and lack of awareness among people will restrain and further impede the growth rate of the market during the forecast period of 2022-2029.
This dermatitis market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the dermatitis market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Patient Epidemiology Analysis
Dermatitis market also provides you with detailed market analysis for patient analysis, prognosis and cures. Prevalence, incidence, mortality, adherence rates are some of the data variables that are available in the report. Direct or indirect impact analyses of epidemiology to market growth are analysed to create a more robust and cohort multivariate statistical model for forecasting the market in the growth period.
COVID-19 Impact on Dermatitis Market
Since its emergence in December 2019, the COVID-19 virus has spread to nearly every country on the planet, prompting the World Health Organization (WHO) to declare it a public health emergency. Due to the financial crisis and the delay in specialty healthcare delivery while prioritizing COVID-19-related treatments, healthcare systems were severely disrupted in the aftermath of the coronavirus pandemic. Patients were unable to see their controls for a variety of reasons, including difficulty accessing a doctor, fear of infection transmission, inability to continue therapies and essential procedures due to pandemic restrictions. Such considerations had a negative impact on the market of dermatitis in recent months.
Recent Development
- In May 2020, Sanofi had announced the U.S. Food and Drug Administration (FDA) approval for Dupixent (dupilumab). It is approved as a first biologic medicine for children aged 6 to 11 years suffering from moderate-to-severe atopic dermatitis. When compared to TCS alone, Dupixent + topical corticosteroids (TCS) resulted in more than twice as many children having clear or nearly clear skin and more than four times as many children having itch relief in the crucial trial. Three-quarters of Dupixent patients saw a 75 percent or greater improvement in total illness, with an average improvement of around 80 percent.
Global Dermatitis Market Scope
The dermatitis market is segmented on the basis of type, treatment, diagnosis, dosage form, route of administration, end-users and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Contact Dermatitis
- Atopic Dermatitis
- Diaper Dermatitis
- Nummular Dermatitis
- Dyshidrotic Dermatitis
- Perioral Dermatitis
- Neurodermatitis
- Seborrheic Dermatitis
- Stasis Dermatitis
Treatment
- Antibiotics
- Antihistamines
- Calcineurin Inhibitors
- Topical Corticosteroids
- Emollients
- Topical Antiseptic
- Others
Diagnosis
- Blood Tests
- Allergy Skin Test
- Skin Biopsy
Dosage form
- Tablets
- Ointments
- Others
Route of Administration
- Oral
- Topical
- Others
End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Dermatitis Market Regional Analysis/Insights
The dermatitis market is analyzed and market size insights and trends are provided by country, type, treatment, diagnosis, dosage form, route of administration, end-users and distribution channel as referenced above.
The countries covered in the dermatitis market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
North America dominates the perioral dermatitis treatment market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the presence of major key players and well-developed healthcare infrastructure in this region.
Asia-Pacific on the other hand is projected to exhibit the highest growth rate during the forecast period of 2022-2029 due to the increasing cases of dermatitis diseases and rising investment in the healthcare sector. Also, growing government support and rising level of disposable income will propel the market's growth rate.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Dermatitis Market Share Analysis
The dermatitis market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to dermatitis market.
Some of the major players operating in the dermatitis market are:
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Mylan N.V. (US)
- Teva Pharmaceutical Industries Ltd.(Israel)
- Sanofi (France), Pfizer Inc. (US)
- GlaxoSmithKline plc (UK)
- Novartis AG (Switzerland)
- AstraZeneca (UK)
- Johnson & Johnson (US)
- Bayer AG (Germany)
- Sun Pharmaceutical Industries Ltd. (India)
- LEO Pharma A/S (Denmark)
- Aurobindo Pharma (India)
- Lupin (India)
- Eli Lilly and Company (US)
- Boehringer Ingelheim International GmbH. (Germany)
- Bausch Health Companies Inc. (Canada)
- Bristol-Myers Squibb Company (US)
- AbbVie Inc. (US)
- Allergan (Ireland)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL DERMATITIS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL DERMATITIS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL DERMATITIS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER'S 5 FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
5.1 PREVALECE OF ATOPIC DERMATITIS, BY COUNTRY
5.2 PREVALECE OF CONTACT DERMATITIS, BY COUNTRY
5.3 PREVALECE OF DYSHIDROTIC DERMATITIS, BY COUNTRY
5.4 PREVALECE OF SEBORRHEIC DERMATITIS, BY COUNTRY
5.5 PREVALECE OF ATOPIC DERMATITIS, BY COUNTRY
5.6 PREVALECE OF STASIS DERMATITIS, BY COUNTRY
5.7 TREATMENT RATE
5.8 MORTALITY RATE
5.9 DRUG ADHERENCE AND THERAPY SWITCH MODEL
5.1 PATEINT TREATMENT SUCCESS RATES
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH DERMATOLOGIST
6.8 OTHER KOL SNAPSHOTS
7 REGULATORY SCENARIO
7.1 FDA APPROVALS
7.2 EMA APPROVALS
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.3.1 BARICITNIB
9.3.2 UPADACITNIB
9.3.3 RUXOLITIIB
9.3.4 ABROCITNIB
9.3.5 NEMOLIZUMAB
9.3.6 LEBRIKIZUMAB
9.3.7 KHK-4083
9.3.8 OTHERS
9.4 PHASE II CANDIDATES
9.4.1 TOFACITNIB
9.4.2 GUSACITNIB
9.4.3 RISANKIZUMAB
9.4.4 BERMEKIMAB
9.4.5 OTHERS
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
10 MARKET OVERVIEW
10.1 DRIVERS
10.2 RESTRAINS
10.3 OPPURTUNITY
10.4 CHALLENGES
11 GLOBAL DERMATITIS MARKET, BY TREATMENT
11.1 OVERVIEW
11.2 PHOSPHODIESTERASE-4 INHIBITORS
11.2.1 BY DRUGS
11.2.1.1. APREMILAST
11.2.1.1.1. MARKET VALUE (USD MN)
11.2.1.1.2. MARKET VOLUME (IU)
11.2.1.1.3. AVERAGE SELLING PRICE (USD)
11.2.1.2. CRISABOROLE
11.2.1.2.1. MARKET VALUE (USD MN)
11.2.1.2.2. MARKET VOLUME (IU)
11.2.1.2.3. AVERAGE SELLING PRICE (USD)
11.2.1.3. ROFLUMILAST
11.2.1.3.1. MARKET VALUE (USD MN)
11.2.1.3.2. MARKET VOLUME (IU)
11.2.1.3.3. AVERAGE SELLING PRICE (USD)
11.2.1.4. OTHERS
11.2.2 BY DRUG TYPE
11.2.2.1. GENERICS
11.2.2.2. BRANDED
11.2.2.2.1. OTEZLA
11.2.2.2.1.1 MARKET VALUE (USD MN)
11.2.2.2.1.2 MARKET VOLUME (IU)
11.2.2.2.1.3 AVERAGE SELLING PRICE (USD)
11.2.2.2.2. EUCRIS
11.2.2.2.2.1 MARKET VALUE (USD MN)
11.2.2.2.2.2 MARKET VOLUME (IU)
11.2.2.2.2.3 AVERAGE SELLING PRICE (USD)
11.2.2.2.3. DAXAS
11.2.2.2.3.1 MARKET VALUE (USD MN)
11.2.2.2.3.2 MARKET VOLUME (IU)
11.2.2.2.3.3 AVERAGE SELLING PRICE (USD)
11.2.2.2.4. OTHERS
11.3 CALCINEURIN INHIBITORS
11.3.1 BY DRUGS
11.3.1.1. TACROLIMUS
11.3.1.1.1. MARKET VALUE (USD MN)
11.3.1.1.2. MARKET VOLUME (IU)
11.3.1.1.3. AVERAGE SELLING PRICE (USD)
11.3.1.2. PIMCROMILUS
11.3.1.2.1. MARKET VALUE (USD MN)
11.3.1.2.2. MARKET VOLUME (IU)
11.3.1.2.3. AVERAGE SELLING PRICE (USD)
11.3.1.3. OTHERS
11.3.2 BY DRUG TYPE
11.3.2.1. GENERICS
11.3.2.2. BRANDED
11.3.2.2.1. PROTOPIC
11.3.2.2.1.1 MARKET VALUE (USD MN)
11.3.2.2.1.2 MARKET VOLUME (IU)
11.3.2.2.1.3 AVERAGE SELLING PRICE (USD)
11.3.2.2.2. ELIDEL
11.3.2.2.2.1 MARKET VALUE (USD MN)
11.3.2.2.2.2 MARKET VOLUME (IU)
11.3.2.2.2.3 AVERAGE SELLING PRICE (USD)
11.3.2.2.3. OTHERS
11.4 BIOLOGICS
11.4.1 DUPLIMAB
11.4.1.1. MARKET VALUE (USD MN)
11.4.1.2. MARKET VOLUME (IU)
11.4.1.3. AVERAGE SELLING PRICE (USD)
11.4.2 TRALOKINUMAB
11.4.2.1. MARKET VALUE (USD MN)
11.4.2.2. MARKET VOLUME (IU)
11.4.2.3. AVERAGE SELLING PRICE (USD)
11.4.3 OTHERS
11.5 SOOTHING PRODUCTS
11.5.1 CALAMINE LOTION
11.5.1.1. MARKET VALUE (USD MN)
11.5.1.2. MARKET VOLUME (IU)
11.5.1.3. AVERAGE SELLING PRICE (USD)
11.5.2 COLLOIDAL OATMEAL
11.5.2.1. MARKET VALUE (USD MN)
11.5.2.2. MARKET VOLUME (IU)
11.5.2.3. AVERAGE SELLING PRICE (USD)
11.5.3 OTHERS
11.6 CORTICOSTEROIDS
11.6.1 BY DRUGS
11.6.1.1. BETAMETHASONE
11.6.1.1.1. MARKET VALUE (USD MN)
11.6.1.1.2. MARKET VOLUME (IU)
11.6.1.1.3. AVERAGE SELLING PRICE (USD)
11.6.1.2. CLOBETASOL
11.6.1.2.1. MARKET VALUE (USD MN)
11.6.1.2.2. MARKET VOLUME (IU)
11.6.1.2.3. AVERAGE SELLING PRICE (USD)
11.6.1.3. FLUOCINONIDE
11.6.1.3.1. MARKET VALUE (USD MN)
11.6.1.3.2. MARKET VOLUME (IU)
11.6.1.3.3. AVERAGE SELLING PRICE (USD)
11.6.1.4. HALOBETASOL
11.6.1.4.1. MARKET VALUE (USD MN)
11.6.1.4.2. MARKET VOLUME (IU)
11.6.1.4.3. AVERAGE SELLING PRICE (USD)
11.6.1.5. FLURANDRENOLIDE
11.6.1.5.1. MARKET VALUE (USD MN)
11.6.1.5.2. MARKET VOLUME (IU)
11.6.1.5.3. AVERAGE SELLING PRICE (USD)
11.6.1.6. AMCINONIDE
11.6.1.6.1. MARKET VALUE (USD MN)
11.6.1.6.2. MARKET VOLUME (IU)
11.6.1.6.3. AVERAGE SELLING PRICE (USD)
11.6.1.7. HYDROCORTISONE
11.6.1.7.1. MARKET VALUE (USD MN)
11.6.1.7.2. MARKET VOLUME (IU)
11.6.1.7.3. AVERAGE SELLING PRICE (USD)
11.6.1.8. TRIAMCINOLONE
11.6.1.8.1. MARKET VALUE (USD MN)
11.6.1.8.2. MARKET VOLUME (IU)
11.6.1.8.3. AVERAGE SELLING PRICE (USD)
11.6.1.9. OTHERS
11.6.2 BY DRUG TYPE
11.6.2.1. GENERICS
11.6.2.2. BRANDED
11.6.2.2.1. CELESTONE
11.6.2.2.1.1 MARKET VALUE (USD MN)
11.6.2.2.1.2 MARKET VOLUME (IU)
11.6.2.2.1.3 AVERAGE SELLING PRICE (USD)
11.6.2.2.2. TEMOVATE
11.6.2.2.2.1 MARKET VALUE (USD MN)
11.6.2.2.2.2 MARKET VOLUME (IU)
11.6.2.2.2.3 AVERAGE SELLING PRICE (USD)
11.6.2.2.3. VANOS
11.6.2.2.3.1 MARKET VALUE (USD MN)
11.6.2.2.3.2 MARKET VOLUME (IU)
11.6.2.2.3.3 AVERAGE SELLING PRICE (USD)
11.6.2.2.4. CYCLOCORT
11.6.2.2.4.1 MARKET VALUE (USD MN)
11.6.2.2.4.2 MARKET VOLUME (IU)
11.6.2.2.4.3 AVERAGE SELLING PRICE (USD)
11.6.2.2.5. ULTRAVATE
11.6.2.2.5.1 MARKET VALUE (USD MN)
11.6.2.2.5.2 MARKET VOLUME (IU)
11.6.2.2.5.3 AVERAGE SELLING PRICE (USD)
11.6.2.2.6. HYDROCORT
11.6.2.2.6.1 MARKET VALUE (USD MN)
11.6.2.2.6.2 MARKET VOLUME (IU)
11.6.2.2.6.3 AVERAGE SELLING PRICE (USD)
11.6.2.2.7. ALPHOSYL
11.6.2.2.7.1 MARKET VALUE (USD MN)
11.6.2.2.7.2 MARKET VOLUME (IU)
11.6.2.2.7.3 AVERAGE SELLING PRICE (USD)
11.6.2.2.8. KENALOG
11.6.2.2.8.1 MARKET VALUE (USD MN)
11.6.2.2.8.2 MARKET VOLUME (IU)
11.6.2.2.8.3 AVERAGE SELLING PRICE (USD)
11.6.2.2.9. ARISTOSPAN
11.6.2.2.9.1 MARKET VALUE (USD MN)
11.6.2.2.9.2 MARKET VOLUME (IU)
11.6.2.2.9.3 AVERAGE SELLING PRICE (USD)
11.6.2.2.10. OTHERS
11.7 ANTIHISTAMINES
11.7.1 BY DRUGS
11.7.1.1. DIPHENHYDRAMINE
11.7.1.1.1. MARKET VALUE (USD MN)
11.7.1.1.2. MARKET VOLUME (IU)
11.7.1.1.3. AVERAGE SELLING PRICE (USD)
11.7.1.2. CHLORPHENIRAMINE
11.7.1.2.1. MARKET VALUE (USD MN)
11.7.1.2.2. MARKET VOLUME (IU)
11.7.1.2.3. AVERAGE SELLING PRICE (USD)
11.7.1.3. CLEMASTINE
11.7.1.3.1. MARKET VALUE (USD MN)
11.7.1.3.2. MARKET VOLUME (IU)
11.7.1.3.3. AVERAGE SELLING PRICE (USD)
11.7.1.4. FEXOFENADINE
11.7.1.4.1. MARKET VALUE (USD MN)
11.7.1.4.2. MARKET VOLUME (IU)
11.7.1.4.3. AVERAGE SELLING PRICE (USD)
11.7.1.5. LORATADINE
11.7.1.5.1. MARKET VALUE (USD MN)
11.7.1.5.2. MARKET VOLUME (IU)
11.7.1.5.3. VERAGE SELLING PRICE (USD)
11.7.1.6. CETRIZINE
11.7.1.6.1. MARKET VALUE (USD MN)
11.7.1.6.2. MARKET VOLUME (IU)
11.7.1.6.3. AVERAGE SELLING PRICE (USD)
11.7.1.7. CLEMASTINE
11.7.1.7.1. MARKET VALUE (USD MN)
11.7.1.7.2. MARKET VOLUME (IU)
11.7.1.7.3. AVERAGE SELLING PRICE (USD)
11.7.1.8. OTHERS
11.7.2 BY DRUG TYPE
11.7.2.1. GENERICS
11.7.2.2. BRANDED
11.7.2.2.1. DYTAN DM
11.7.2.2.1.1 MARKET VALUE (USD MN)
11.7.2.2.1.2 MARKET VOLUME (IU)
11.7.2.2.1.3 AVERAGE SELLING PRICE (USD)
11.7.2.2.2. CHLOR TRIMETON
11.7.2.2.2.1 MARKET VALUE (USD MN)
11.7.2.2.2.2 MARKET VOLUME (IU)
11.7.2.2.2.3 AVERAGE SELLING PRICE (USD)
11.7.2.2.3. TAVIST ALLERGY
11.7.2.2.3.1 MARKET VALUE (USD MN)
11.7.2.2.3.2 MARKET VOLUME (IU)
11.7.2.2.3.3 AVERAGE SELLING PRICE (USD)
11.7.2.2.4. ALLEGRA
11.7.2.2.4.1 MARKET VALUE (USD MN)
11.7.2.2.4.2 MARKET VOLUME (IU)
11.7.2.2.4.3 AVERAGE SELLING PRICE (USD)
11.7.2.2.5. CLARITIN
11.7.2.2.5.1 MARKET VALUE (USD MN)
11.7.2.2.5.2 MARKET VOLUME (IU)
11.7.2.2.5.3 AVERAGE SELLING PRICE (USD)
11.7.2.2.6. ZYRTEC
11.7.2.2.6.1 MARKET VALUE (USD MN)
11.7.2.2.6.2 MARKET VOLUME (IU)
11.7.2.2.6.3 AVERAGE SELLING PRICE (USD)
11.7.2.2.7. OTHERS
11.8 ANTIBIOTICS
11.8.1 BY DRUGS
11.8.1.1. ERYTHROMYCIN
11.8.1.1.1. MARKET VALUE (USD MN)
11.8.1.1.2. MARKET VOLUME (IU)
11.8.1.1.3. AVERAGE SELLING PRICE (USD)
11.8.1.2. SULFACETAMIDE
11.8.1.2.1. MARKET VALUE (USD MN)
11.8.1.2.2. MARKET VOLUME (IU)
11.8.1.2.3. AVERAGE SELLING PRICE (USD)
11.8.1.3. NEOMYCIN
11.8.1.3.1. MARKET VALUE (USD MN)
11.8.1.3.2. MARKET VOLUME (IU)
11.8.1.3.3. AVERAGE SELLING PRICE (USD)
11.8.1.4. VANCOMYCIN
11.8.1.4.1. MARKET VALUE (USD MN)
11.8.1.4.2. MARKET VOLUME (IU)
11.8.1.4.3. AVERAGE SELLING PRICE (USD)
11.8.1.5. LINEZOLID
11.8.1.5.1. MARKET VALUE (USD MN)
11.8.1.5.2. MARKET VOLUME (IU)
11.8.1.5.3. AVERAGE SELLING PRICE (USD)
11.8.1.6. CEFTAROLINE
11.8.1.6.1. MARKET VALUE (USD MN)
11.8.1.6.2. MARKET VOLUME (IU)
11.8.1.6.3. AVERAGE SELLING PRICE (USD)
11.8.1.7. DAPTOMYCIN
11.8.1.7.1. MARKET VALUE (USD MN)
11.8.1.7.2. MARKET VOLUME (IU)
11.8.1.7.3. AVERAGE SELLING PRICE (USD)
11.8.1.8. OFLOXACIN
11.8.1.8.1. MARKET VALUE (USD MN)
11.8.1.8.2. MARKET VOLUME (IU)
11.8.1.8.3. AVERAGE SELLING PRICE (USD)
11.8.1.9. CIPROFLOXACIN
11.8.1.9.1. MARKET VALUE (USD MN)
11.8.1.9.2. MARKET VOLUME (IU)
11.8.1.9.3. AVERAGE SELLING PRICE (USD)
11.8.1.10. OTHERS
11.8.2 BY DRUG TYPE
11.8.2.1. GENERICS
11.8.2.2. BRANDED
11.8.2.2.1. KLARON
11.8.2.2.1.1 MARKET VALUE (USD MN)
11.8.2.2.1.2 MARKET VOLUME (IU)
11.8.2.2.1.3 AVERAGE SELLING PRICE (USD)
11.8.2.2.2. OVACE
11.8.2.2.2.1 MARKET VALUE (USD MN)
11.8.2.2.2.2 MARKET VOLUME (IU)
11.8.2.2.2.3 AVERAGE SELLING PRICE (USD)
11.8.2.2.3. VANCOCIN
11.8.2.2.3.1 MARKET VALUE (USD MN)
11.8.2.2.3.2 MARKET VOLUME (IU)
11.8.2.2.3.3 AVERAGE SELLING PRICE (USD)
11.8.2.2.4. ZYVOX
11.8.2.2.4.1 MARKET VALUE (USD MN)
11.8.2.2.4.2 MARKET VOLUME (IU)
11.8.2.2.4.3 AVERAGE SELLING PRICE (USD)
11.8.2.2.5. TEFLARO
11.8.2.2.5.1 MARKET VALUE (USD MN)
11.8.2.2.5.2 MARKET VOLUME (IU)
11.8.2.2.5.3 AVERAGE SELLING PRICE (USD)
11.8.2.2.6. CUBICIN
11.8.2.2.6.1 MARKET VALUE (USD MN)
11.8.2.2.6.2 MARKET VOLUME (IU)
11.8.2.2.6.3 AVERAGE SELLING PRICE (USD)
11.8.2.2.7. FLOXIN
11.8.2.2.7.1 MARKET VALUE (USD MN)
11.8.2.2.7.2 MARKET VOLUME (IU)
11.8.2.2.7.3 AVERAGE SELLING PRICE (USD)
11.8.2.2.8. CIPRO
11.8.2.2.8.1 MARKET VALUE (USD MN)
11.8.2.2.8.2 MARKET VOLUME (IU)
11.8.2.2.8.3 AVERAGE SELLING PRICE (USD)
11.8.2.2.9. OTHERS
12 GLOBAL DERMATITIS MARKET, BY ROUTE OF ADMINISTRATION
12.1 OVERVIEW
12.2 TOPICAL
12.2.1 CREAM
12.2.2 LOTION
12.2.3 OINTMENT
12.2.4 SPRAY
12.2.5 OTHERS
12.3 ORAL
12.3.1 TABLET
12.3.2 CALPSULES
12.3.3 SYRUP
12.3.4 OTHERS
12.4 OTHERS
12.5 RECONSTITUTE
13 GLOBAL DERMATITIS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITALS
13.3 DERMATOLOGY CLINICS
13.4 ACADEMIC AND RESEARCH INSTITUTES
13.5 OTHERS
14 GLOBAL DERMATITIS MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 HOSPITAL PHARMACY
14.3 ONLINE PHARMACY
14.4 RETAIL PHARMACY
15 GLOBAL DERMATITIS MARKET, BY GEOGRAPHY
15.1 GLOBAL DERMATITIS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
15.2 NORTH AMERICA
15.2.1 U.S.
15.2.1.1. U.S. DERMATITIS MARKET, BY TREATMENT TYPE
15.2.1.2. U.S. DERMATITIS MARKET, BY ROUTE OF ADMINISTRATION
15.2.1.3. U.S. DERMATITIS MARKET, BY END USER
15.2.1.4. U.S. DERMATITIS MARKET, BY DISTRIBUTION CHANNEL
15.2.2 CANADA
15.2.3 MEXICO
15.2.4 DOMINICAN REPUBLIC
15.2.5 JAMAICA
15.2.6 PANAMA
15.3 EUROPE
15.3.1 GERMANY
15.3.2 FRANCE
15.3.3 U.K.
15.3.4 HUNGARY
15.3.5 LITHUANIA
15.3.6 AUSTRIA
15.3.7 IRELAND
15.3.8 NORWAY
15.3.9 POLAND
15.3.10 ITALY
15.3.11 SPAIN
15.3.12 RUSSIA
15.3.13 TURKEY
15.3.14 NETHERLANDS
15.3.15 SWITZERLAND
15.3.16 REST OF EUROPE
15.4 ASIA-PACIFIC
15.4.1 JAPAN
15.4.2 CHINA
15.4.3 TAIWAN
15.4.4 SOUTH KOREA
15.4.5 INDIA
15.4.6 AUSTRALIA
15.4.7 SINGAPORE
15.4.8 THAILAND
15.4.9 MALAYSIA
15.4.10 INDONESIA
15.4.11 PHILIPPINES
15.4.12 VIETNAM
15.4.13 REST OF ASIA-PACIFIC
15.5 SOUTH AMERICA
15.5.1 BRAZIL
15.5.2 ECUADOR
15.5.3 CHILE
15.5.4 COLOMBIA
15.5.5 VENEZUELA
15.5.6 ARGENTINA
15.5.7 PERU
15.5.8 CURAÇAO
15.5.9 PARAGUAY
15.5.10 URUGUAY
15.5.11 TRINIDAD AND TOBAGO
15.5.12 REST OF SOUTH AMERICA
15.6 MIDDLE EAST AND AFRICA
15.6.1 SOUTH AFRICA
15.6.2 SAUDI ARABIA
15.6.3 UAE
15.6.4 EGYPT
15.6.5 KUWAIT
15.6.6 ISRAEL
15.6.7 BOLIVIA
15.6.8 REST OF MIDDLE EAST AND AFRICA
15.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
16 GLOBAL DERMATITIS MARKET, SWOT AND DBMR ANALYSIS
17 GLOBAL DERMATITIS MARKET, COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: GLOBAL
17.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
17.3 COMPANY SHARE ANALYSIS: EUROPE
17.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
17.5 MERGERS & ACQUISITIONS
17.6 NEW PRODUCT DEVELOPMENT & APPROVALS
17.7 EXPANSIONS
17.8 REGULATORY CHANGES
17.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
18 GLOBAL DERMATITIS MARKET, COMPANY PROFILE
18.1 PFIZER, INC
18.1.1 COMPANY OVERVIEW
18.1.2 REVENUE ANALYSIS
18.1.3 GEOGRAPHIC PRESENCE
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENTS
18.2 JOHNSON & JOHNSON (JANSSEN PHARMACEUTICALS)
18.2.1 COMPANY OVERVIEW
18.2.2 REVENUE ANALYSIS
18.2.3 GEOGRAPHIC PRESENCE
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 NOVARTIS AG
18.3.1 COMPANY OVERVIEW
18.3.2 REVENUE ANALYSIS
18.3.3 GEOGRAPHIC PRESENCE
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENTS
18.4 AMGEN
18.4.1 COMPANY OVERVIEW
18.4.2 REVENUE ANALYSIS
18.4.3 GEOGRAPHIC PRESENCE
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENTS
18.5 SANOFI
18.5.1 COMPANY OVERVIEW
18.5.2 REVENUE ANALYSIS
18.5.3 GEOGRAPHIC PRESENCE
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENTS
18.6 BRISTOL MYERS SQUIBB
18.6.1 COMPANY OVERVIEW
18.6.2 REVENUE ANALYSIS
18.6.3 GEOGRAPHIC PRESENCE
18.6.4 PRODUCT PORTFOLIO
18.6.5 RECENT DEVELOPMENTS
18.7 BAYER AG
18.7.1 COMPANY OVERVIEW
18.7.2 REVENUE ANALYSIS
18.7.3 GEOGRAPHIC PRESENCE
18.7.4 PRODUCT PORTFOLIO
18.7.5 RECENT DEVELOPMENTS
18.8 ASTELLAS PHARMA INC
18.8.1 COMPANY OVERVIEW
18.8.2 REVENUE ANALYSIS
18.8.3 GEOGRAPHIC PRESENCE
18.8.4 PRODUCT PORTFOLIO
18.8.5 RECENT DEVELOPMENTS
18.9 REGENERON PHARMACEUTICALS, INC
18.9.1 COMPANY OVERVIEW
18.9.2 REVENUE ANALYSIS
18.9.3 GEOGRAPHIC PRESENCE
18.9.4 PRODUCT PORTFOLIO
18.9.5 RECENT DEVELOPMENTS
18.1 F-HOFFMANN LA ROCHE
18.10.1 COMPANY OVERVIEW
18.10.2 REVENUE ANALYSIS
18.10.3 GEOGRAPHIC PRESENCE
18.10.4 PRODUCT PORTFOLIO
18.10.5 RECENT DEVELOPMENTS
18.11 GLAXOSMITHKLINE PLC
18.11.1 COMPANY OVERVIEW
18.11.2 REVENUE ANALYSIS
18.11.3 GEOGRAPHIC PRESENCE
18.11.4 PRODUCT PORTFOLIO
18.11.5 RECENT DEVELOPMENTS
18.12 MERCK SHARP & DOHME CORP
18.12.1 COMPANY OVERVIEW
18.12.2 REVENUE ANALYSIS
18.12.3 GEOGRAPHIC PRESENCE
18.12.4 PRODUCT PORTFOLIO
18.12.5 RECENT DEVELOPMENTS
18.13 MYLAN NV
18.13.1 COMPANY OVERVIEW
18.13.2 REVENUE ANALYSIS
18.13.3 GEOGRAPHIC PRESENCE
18.13.4 PRODUCT PORTFOLIO
18.13.5 RECENT DEVELOPMENTS
18.14 TEVA PHARMACEUTICALS
18.14.1 COMPANY OVERVIEW
18.14.2 REVENUE ANALYSIS
18.14.3 GEOGRAPHIC PRESENCE
18.14.4 PRODUCT PORTFOLIO
18.14.5 RECENT DEVELOPMENTS
18.15 ASTRAZENCA
18.15.1 COMPANY OVERVIEW
18.15.2 REVENUE ANALYSIS
18.15.3 GEOGRAPHIC PRESENCE
18.15.4 PRODUCT PORTFOLIO
18.15.5 RECENT DEVELOPMENTS
18.16 AKORN
18.16.1 COMPANY OVERVIEW
18.16.2 REVENUE ANALYSIS
18.16.3 GEOGRAPHIC PRESENCE
18.16.4 PRODUCT PORTFOLIO
18.16.5 RECENT DEVELOPMENTS
18.17 BOEHRINGER INGELHEIM INTERNATIONAL GMBH
18.17.1 COMPANY OVERVIEW
18.17.2 REVENUE ANALYSIS
18.17.3 GEOGRAPHIC PRESENCE
18.17.4 PRODUCT PORTFOLIO
18.17.5 RECENT DEVELOPMENTS
18.18 CIPLA PHARMACEUTICALS
18.18.1 COMPANY OVERVIEW
18.18.2 REVENUE ANALYSIS
18.18.3 GEOGRAPHIC PRESENCE
18.18.4 PRODUCT PORTFOLIO
18.18.5 RECENT DEVELOPMENTS
18.19 OTSUKA PHARMACEUTICALS
18.19.1 COMPANY OVERVIEW
18.19.2 REVENUE ANALYSIS
18.19.3 GEOGRAPHIC PRESENCE
18.19.4 PRODUCT PORTFOLIO
18.19.5 RECENT DEVELOPMENTS
18.2 GALDERMA
18.20.1 COMPANY OVERVIEW
18.20.2 REVENUE ANALYSIS
18.20.3 GEOGRAPHIC PRESENCE
18.20.4 PRODUCT PORTFOLIO
18.20.5 RECENT DEVELOPMENTS
18.21 ARENA PHARMACEUTICALS
18.21.1 COMPANY OVERVIEW
18.21.2 REVENUE ANALYSIS
18.21.3 GEOGRAPHIC PRESENCE
18.21.4 PRODUCT PORTFOLIO
18.21.5 RECENT DEVELOPMENTS
18.22 ASANA BIOSCIENCES
18.22.1 COMPANY OVERVIEW
18.22.2 REVENUE ANALYSIS
18.22.3 GEOGRAPHIC PRESENCE
18.22.4 PRODUCT PORTFOLIO
18.22.5 RECENT DEVELOPMENTS
18.23 ZYDUS HEALTHCARE
18.23.1 COMPANY OVERVIEW
18.23.2 REVENUE ANALYSIS
18.23.3 GEOGRAPHIC PRESENCE
18.23.4 PRODUCT PORTFOLIO
18.23.5 RECENT DEVELOPMENTS
18.24 ABBVIE
18.24.1 COMPANY OVERVIEW
18.24.2 REVENUE ANALYSIS
18.24.3 GEOGRAPHIC PRESENCE
18.24.4 PRODUCT PORTFOLIO
18.24.5 RECENT DEVELOPMENTS
18.25 GENZYME PHARMA
18.25.1 COMPANY OVERVIEW
18.25.2 REVENUE ANALYSIS
18.25.3 GEOGRAPHIC PRESENCE
18.25.4 PRODUCT PORTFOLIO
18.25.5 RECENT DEVELOPMENTS
18.26 GILEAD SCIENCES
18.26.1 COMPANY OVERVIEW
18.26.2 REVENUE ANALYSIS
18.26.3 GEOGRAPHIC PRESENCE
18.26.4 PRODUCT PORTFOLIO
18.26.5 RECENT DEVELOPMENTS
18.27 ELI LILLY
18.27.1 COMPANY OVERVIEW
18.27.2 REVENUE ANALYSIS
18.27.3 GEOGRAPHIC PRESENCE
18.27.4 PRODUCT PORTFOLIO
18.27.5 RECENT DEVELOPMENTS
18.28 TAKEDA PHARMACEUTICALS
18.28.1 COMPANY OVERVIEW
18.28.2 REVENUE ANALYSIS
18.28.3 GEOGRAPHIC PRESENCE
18.28.4 PRODUCT PORTFOLIO
18.28.5 RECENT DEVELOPMENTS
18.29 UCB PHARMA
18.29.1 COMPANY OVERVIEW
18.29.2 REVENUE ANALYSIS
18.29.3 GEOGRAPHIC PRESENCE
18.29.4 PRODUCT PORTFOLIO
18.29.5 RECENT DEVELOPMENTS
18.3 EISAI CO
18.30.1 COMPANY OVERVIEW
18.30.2 REVENUE ANALYSIS
18.30.3 GEOGRAPHIC PRESENCE
18.30.4 PRODUCT PORTFOLIO
18.30.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
19 RELATED REPORTS
20 CONCLUSION
21 QUESTIONNAIRE
22 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.