Global Dermatome Device Market
Market Size in USD Million
CAGR :
% 
 USD
226.98 Million
USD
350.19 Million
2025
2033
USD
226.98 Million
USD
350.19 Million
2025
2033
| 2026 –2033 | |
| USD 226.98 Million | |
| USD 350.19 Million | |
|
|
|
|
Dermatome Device Market Size
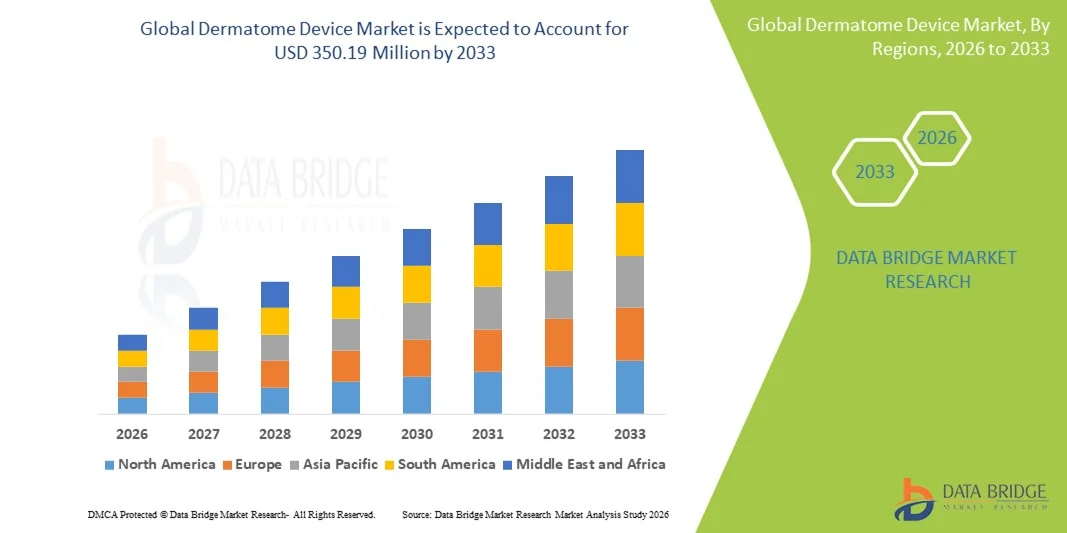
- The global dermatome device market size was valued at USD 226.98 million in 2025 and is expected to reach USD 350.19 million by 2033, at a CAGR of 5.57% during the forecast period
- The market growth is largely fueled by the increasing number of burn injuries, chronic wounds, and skin trauma worldwide, which drive demand for skin grafting procedures where dermatomes are essential tools. In addition, the rising volume of cosmetic and reconstructive surgeries globally is contributing to expanded adoption of dermatome devices
- Furthermore, technological advancements in device precision, rising consumer demand for minimally invasive and accurate surgical tools, and expanding healthcare infrastructure in developing regions are establishing dermatome devices as critical instruments in wound management and plastic surgical procedures. These converging factors are accelerating the uptake of dermatome solutions, thereby significantly boosting the industry’s growth
Dermatome Device Market Analysis
- Dermatome devices, used for harvesting precise skin grafts in reconstructive and burn surgeries, are increasingly essential tools in modern surgical and wound care practices due to their accuracy, efficiency, and ability to minimize tissue damage
- The escalating demand for dermatome devices is primarily fueled by the rising incidence of burn injuries, chronic wounds, and skin trauma worldwide, coupled with growing volumes of cosmetic and reconstructive surgeries and an increasing preference for minimally invasive and precise surgical tools
- North America dominated the dermatome device market with the largest revenue share of 38.7% in 2025, driven by advanced healthcare infrastructure, high adoption of innovative surgical devices, and the presence of major market players. The U.S. leads in dermatome device usage, particularly in burn units and plastic surgery centers, supported by continuous technological advancements and surgeon preference for precision instruments
- Asia-Pacific is expected to be the fastest growing region in the dermatome device market during the forecast period due to rising healthcare investments, expanding hospital networks, increasing awareness of advanced wound care, and a growing number of reconstructive and cosmetic procedures
- Powered dermatome segment dominated the market with a market share of 46.5% in 2025, driven by its precision, consistent graft thickness, and efficiency
Report Scope and Dermatome Device Market Segmentation
|
Attributes |
Dermatome Device Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Dermatome Device Market Trends
Advancements in Precision and Automated Dermatomes
- A key and accelerating trend in the global dermatome device market is the development of precision-powered and automated dermatomes that enable consistent skin graft thickness, reducing surgical time and improving patient outcomes
- For instance, powered dermatomes from companies such as Zimmer Biomet and Aesculap offer adjustable depth control and automated harvesting, allowing surgeons to perform grafting procedures with higher accuracy and minimal manual effort
- Integration with digital imaging and measurement systems allows dermatomes to provide real-time feedback on graft thickness and area, improving procedural efficiency and minimizing tissue waste
- These technological advancements facilitate improved workflow in operating rooms, especially in burn units and reconstructive surgery centers, where precision and speed are critical
- The trend towards more automated, accurate, and surgeon-friendly dermatome devices is shaping user expectations, with medical institutions seeking solutions that enhance procedural safety and outcomes
- The demand for dermatomes that combine precision, automation, and ease of use is increasing globally, as hospitals and specialty clinics prioritize patient safety, procedural efficiency, and reduced operative variability
- Expanding adoption of portable and battery-operated dermatomes is enabling faster deployment in emergency burn units and field hospitals, creating new usage opportunities beyond traditional operating rooms
Dermatome Device Market Dynamics
Driver
Increasing Incidence of Burns and Reconstructive Surgeries
- The rising prevalence of burn injuries, chronic wounds, and reconstructive surgeries worldwide is a major driver for the growing demand for dermatome devices
- For instance, Zimmer Biomet reported increased adoption of its powered dermatomes in burn centers due to a surge in skin graft procedures and reconstructive interventions
- As healthcare providers focus on improving patient outcomes and reducing operative complications, dermatome devices provide precision harvesting and consistent graft quality, essential for successful grafting
- Furthermore, the growing number of cosmetic and plastic surgeries is driving adoption in hospitals and specialty clinics, reflecting a broader need for advanced skin grafting solutions
- The demand for minimally invasive and efficient surgical instruments is increasing, making dermatomes a critical component of modern surgical practice
- The expansion of healthcare infrastructure and investment in advanced surgical tools in emerging economies is creating new markets for dermatome devices
- Increased awareness and training programs for surgeons on the benefits of automated dermatomes are accelerating adoption and driving overall market growth
Restraint/Challenge
High Device Cost and Training Requirements
- The relatively high cost of advanced powered dermatomes compared to manual options poses a challenge for adoption, particularly in developing regions and smaller clinics
- For instance, the investment required for automated dermatome systems from companies such as Aesculap can be prohibitive for budget-conscious hospitals and ambulatory surgical centers
- In addition, specialized training is required for surgeons and operating room staff to operate these devices safely and effectively, which can slow adoption
- Smaller healthcare facilities may hesitate to invest in these devices due to operational costs, maintenance, and the learning curve associated with precision dermatomes
- Overcoming these challenges through cost-effective device models, surgeon training programs, and demonstrations of improved patient outcomes will be crucial for sustained market growth
- Concerns about device maintenance and blade replacement costs may limit adoption in resource-constrained facilities
- Regulatory approvals and compliance requirements for powered dermatomes across different regions can delay market entry and adoption for new product launches
Dermatome Device Market Scope
The market is segmented on the basis of type and end-user.
- By Type
On the basis of type, the dermatome device market is segmented into drum dermatome, air dermatome, knife dermatome, and powered dermatome. Powered Dermatome segment dominated the market with the largest market revenue share of 46.5% in 2025, driven by its precision, consistent graft thickness, and ability to reduce manual effort during surgeries. Hospitals and burn centers prefer powered dermatomes due to their reliability and ability to perform high-volume grafting procedures efficiently. The automation and adjustable depth control features allow surgeons to maintain uniform graft thickness, improving patient outcomes and reducing complications. The powered dermatome is also preferred for reconstructive and cosmetic surgeries, where precision and speed are critical. Technological advancements, including digital integration and improved blade systems, have further strengthened its market position. In addition, powered dermatomes are increasingly adopted in emerging markets due to expanding healthcare infrastructure and growing numbers of reconstructive surgery procedures.
Knife Dermatome segment is anticipated to witness the fastest growth rate of 20.3% from 2026 to 2033, fueled by its simplicity, lower cost, and portability. Knife dermatomes are widely used in smaller clinics and ambulatory surgical centers where high-end powered devices may not be feasible. Surgeons value knife dermatomes for their ease of sterilization and maneuverability in smaller operating rooms. The device’s low maintenance requirements and adaptability to various graft thicknesses make it suitable for developing regions. Increasing awareness of reconstructive and cosmetic surgery procedures in emerging markets is boosting the adoption of knife dermatomes. Moreover, training programs for surgeons and improved availability of disposable blades contribute to their accelerated adoption.
- By End User
On the basis of end user, the dermatome device market is segmented into hospitals, ambulatory surgical centres, and specialty clinics. Hospitals segment dominated the market with a market share of 62.1% in 2025, owing to the high volume of burn care and reconstructive surgeries performed in hospital settings. Hospitals have advanced surgical infrastructure, trained surgeons, and established protocols that favor the use of precision dermatomes, especially powered models. The need for consistent graft quality, reduced operative time, and higher patient throughput further strengthens hospitals’ dominance. Large-scale adoption is also driven by investments in modern burn units and plastic surgery departments. Hospitals benefit from better post-operative care facilities, making them ideal end-users for high-end dermatome devices. In addition, hospitals in developed regions continue to upgrade their equipment with advanced powered dermatomes to enhance surgical efficiency and patient outcomes.
Ambulatory Surgical Centres (ASCs) are expected to witness the fastest growth rate of 18.7% from 2026 to 2033, driven by the increasing number of minor reconstructive, cosmetic, and outpatient procedures. ASCs provide cost-effective and convenient alternatives to hospital-based surgeries, making them attractive for elective cosmetic procedures. The adoption of portable and user-friendly knife and air dermatomes is accelerating in ASCs due to space and cost constraints. Surgeons and patients prefer ASCs for quick procedures with shorter recovery times, which aligns with the benefits offered by compact dermatome devices. The rising trend of outpatient cosmetic surgeries in both developed and emerging markets is contributing to rapid growth. Furthermore, technological improvements in portable dermatomes make them highly compatible with ASC environments, encouraging adoption.
Dermatome Device Market Regional Analysis
- North America dominated the dermatome device market with the largest revenue share of 38.7% in 2025, driven by advanced healthcare infrastructure, high adoption of innovative surgical devices, and the presence of major market players
- Healthcare providers and hospitals in the region prioritize precision and efficiency in skin grafting procedures, making powered dermatomes the preferred choice for reconstructive and cosmetic surgeries
- This widespread adoption is further supported by the presence of key market players, high surgeon awareness and training programs, and continuous investments in modern burn care and plastic surgery centers, establishing dermatomes as essential instruments in surgical practices for hospitals, specialty clinics, and burn units
U.S. Dermatome Device Market Insight
The U.S. dermatome device market captured the largest revenue share of 82% in 2025 within North America, driven by the high prevalence of burn injuries and reconstructive surgeries. Hospitals and specialty clinics increasingly prioritize powered dermatomes for precision graft harvesting and efficiency in high-volume procedures. The growing number of cosmetic and plastic surgeries, coupled with advanced healthcare infrastructure and trained surgical staff, further supports market expansion. In addition, the demand for minimally invasive and automated dermatome solutions is rising due to patient safety and improved procedural outcomes. The U.S. also benefits from active R&D and the presence of major market players introducing innovative devices, which strengthens adoption. Moreover, reimbursement policies for reconstructive surgeries facilitate broader usage of high-end dermatome systems across hospitals and clinics.
Europe Dermatome Device Market Insight
The Europe dermatome device market is projected to expand at a substantial CAGR during the forecast period, primarily driven by increasing awareness of burn care management and reconstructive surgery standards. The rise in hospital investments and specialized surgical centers is fostering adoption of powered and precision dermatomes. European healthcare providers prioritize procedural efficiency, graft consistency, and safety, which are key advantages of modern dermatome devices. Furthermore, government initiatives promoting advanced surgical equipment and training programs for surgeons are supporting market growth. The region also shows growing demand across hospitals, ambulatory surgical centers, and specialty clinics, reflecting a broader acceptance of dermatomes for both new and existing surgical setups. Consumers and medical professionals value dermatomes for improved patient outcomes and reduced operative time, particularly in high-demand reconstructive procedures.
U.K. Dermatome Device Market Insight
The U.K. dermatome device market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing adoption of powered dermatomes in hospitals and specialty clinics. Rising awareness of burn care, cosmetic surgery, and skin graft procedures encourages medical facilities to invest in precision dermatomes. In addition, government-supported training programs and safety standards promote the use of advanced dermatome devices. The U.K.’s healthcare infrastructure, combined with high surgeon expertise and patient preference for minimally invasive procedures, is expected to continue driving market growth. The focus on consistent graft thickness, reduced operative time, and improved patient outcomes makes dermatome devices highly valued. Moreover, the integration of dermatomes into modern surgical workflows is increasing across reconstructive and elective surgeries.
Germany Dermatome Device Market Insight
The Germany dermatome device market is expected to expand at a considerable CAGR during the forecast period, fueled by rising investments in healthcare infrastructure and growing emphasis on reconstructive and cosmetic procedures. Hospitals and burn centers prioritize precision, safety, and efficiency, making powered dermatomes the preferred choice. Germany’s emphasis on innovation and adoption of advanced medical devices promotes the uptake of automated dermatomes. Furthermore, surgeons increasingly rely on these devices for consistent graft harvesting and reduced intraoperative complications. The demand for dermatomes is also driven by patient expectations for improved outcomes and minimal recovery times. Technological advancements, such as integration with digital imaging and adjustable blade systems, support continued adoption across hospitals and specialty clinics.
Asia-Pacific Dermatome Device Market Insight
The Asia-Pacific dermatome device market is poised to grow at the fastest CAGR of 25% during 2026–2033, driven by increasing burn cases, expanding reconstructive surgeries, and rising healthcare investments in countries such as China, Japan, and India. The region’s growing hospital networks, specialty clinics, and ambulatory surgical centers are adopting powered and knife dermatomes to improve grafting efficiency. Government initiatives promoting healthcare modernization, training programs for surgeons, and rising awareness of cosmetic procedures support adoption. In addition, the manufacturing of dermatome devices locally reduces costs, making advanced devices more accessible. The increasing number of cosmetic surgeries and skin graft procedures further fuels the demand. Growing urbanization and rising disposable incomes enable wider penetration of dermatome devices across both public and private healthcare sectors.
Japan Dermatome Device Market Insight
The Japan dermatome device market is gaining momentum due to the country’s advanced healthcare infrastructure, high surgical standards, and growing demand for precision in reconstructive and cosmetic procedures. Hospitals and specialty clinics focus on powered dermatomes for consistent graft thickness and efficiency. The rising number of cosmetic surgeries and an aging population drive demand for devices that simplify procedures and reduce operative complications. Integration with digital surgical tools enhances accuracy and workflow in operating rooms. Moreover, medical professionals value dermatomes for minimizing tissue damage and improving patient outcomes. Government support for advanced surgical equipment adoption also accelerates market growth.
India Dermatome Device Market Insight
The India dermatome device market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to rapid urbanization, a growing middle class, and increased healthcare spending. Hospitals, ambulatory surgical centers, and specialty clinics are increasingly adopting powered and knife dermatomes to meet the rising demand for burn care and reconstructive surgeries. The push towards modernizing healthcare infrastructure and initiatives for cosmetic surgery awareness supports market growth. Affordable device options and local manufacturing of dermatomes make them more accessible to a wider range of healthcare facilities. The expanding number of cosmetic procedures and skin graft surgeries drives further adoption. Moreover, rising surgeon training programs and awareness of advanced dermatome technology contribute to faster market penetration.
Dermatome Device Market Share
The Dermatome Device industry is primarily led by well-established companies, including:
- Zimmer Biomet (U.S.)
- Integra LifeSciences Corporation (U.S.)
- B. Braun SE (Germany)
- Aesculap, Inc. (U.S.)
- Surtex Instruments (U.K.)
- De Soutter Medical (U.K.)
- Humeca (Netherlands)
- Nouvag AG (Switzerland)
- Exsurco Medical, Inc. (U.S.)
- A.P. Surgical Industries (U.S.)
- Aygun Surgical Instruments Co., Inc. (U.S.)
- Weigao Group (China)
- Shanghai Medical Instruments Ltd. (China)
- Ermis MedTech (Turkey)
- Yuanhua Medical (China)
- Ranfac Corp. (U.S.)
- Delacroix Chevalier (France)
- Medin Technologies (Czech Republic)
- SteriLance Medical Inc. (China)
- Millennium Surgical Corp. (U.S.)
What are the Recent Developments in Global Dermatome Device Market?
- In January 2026, researchers highlighted advances in bio‑engineered skin grafts offering new hope for treating severe burn injuries, noting clinical progress in producing living skin tissue grafts from a patient’s own cells, potentially impacting future grafting approaches and minimizing reliance on traditional dermatomes
- In February 2025, CUTISS reported positive Phase 2 clinical trial results for denovoSkin™, showing promising outcomes in reconstructive and burn surgery with reduced need for traditional graft harvesting and improved patient recovery
- In October 2024, CUTISS AG announced the first ever use of its bio‑engineered denovoSkin™ graft in a compassionate reconstructive surgery in the U.S. for a pediatric burn patient, marking a significant clinical milestone in advanced grafting and reconstructive skin technology
- In March 2024, the U.S. Food and Drug Administration (FDA) authorized marketing of the Medline Autologous Regeneration of Tissue (ART) Skin Harvesting System, a first‑of‑its‑kind semi‑automated handheld device that harvests skin micrografts and applies them directly to wounds in a minimally invasive way, expanding clinical use beyond traditional grafting procedures
- In March 2024, detailed regulatory documentation showed the FDA’s De Novo classification for the Medline ART Skin Harvesting System, confirming it as a novel semi‑automated device category for skin graft harvesting and application a foundational regulatory milestone enabling broader clinical adoption
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.