Global Dysmenorrhea Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
5,937.80 Million
USD
11,658.88 Million
2022
2030
USD
5,937.80 Million
USD
11,658.88 Million
2022
2030
| 2023 –2030 | |
| USD 5,937.80 Million | |
| USD 11,658.88 Million | |
|
|
|
|
Dysmenorrhea Treatment Market Analysis and Size
The dysmenorrhea treatment market is expanding as a result two primary key factors such as rising consumer awareness of dysmenorrhea therapy and the low price of over-the-counter drugs. As per the article published in the Women's Health Concern and British Menopause Society in December 2020, around 80% of dysmenorrhea women experience period pain at some stage in their lifetime. The launch of several new products for treating the pain related with primary and secondary dysmenorrhea is also pushing the market.
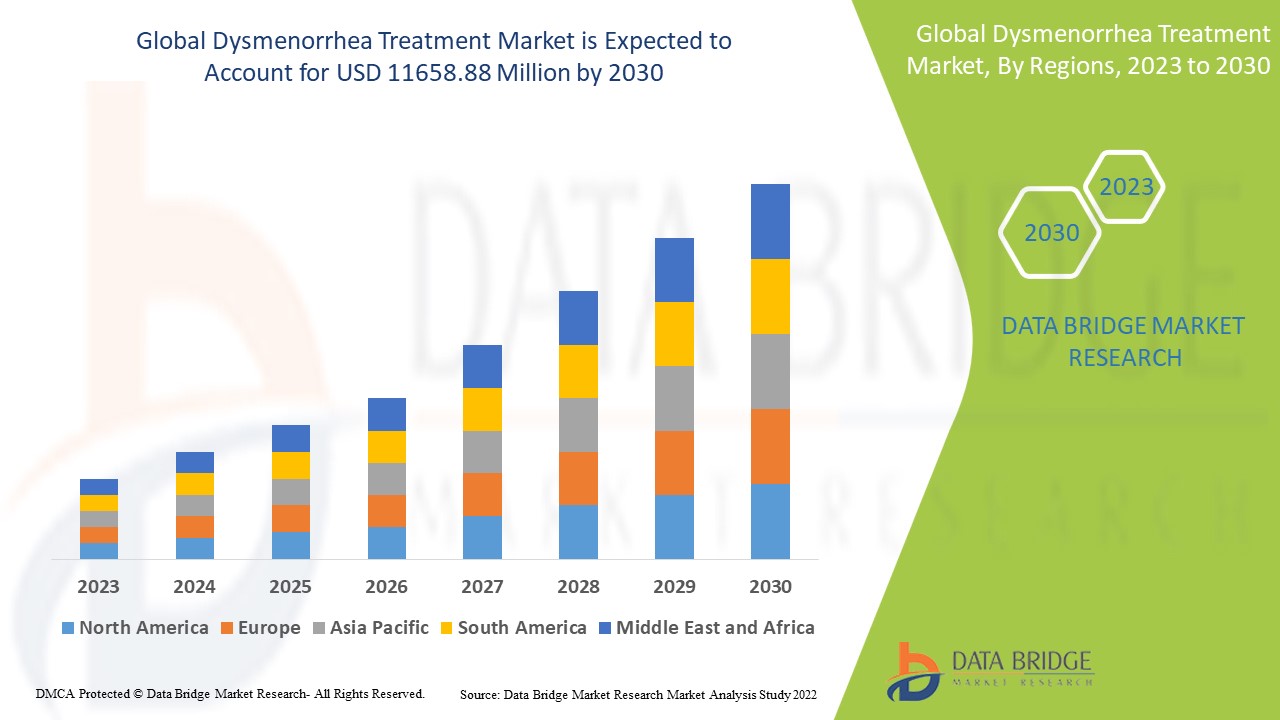
Data Bridge Market Research analyses a growth rate in the dysmenorrhea treatment market in the forecast period 2023-2030. The expected CAGR of dysmenorrhea treatment market is tend to be around 8.8% in the mentioned forecast period. The market was valued at USD 5937.8 million in 2022, and it would grow upto USD 11658.88 million by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Dysmenorrhea Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Primary Dysmenorrhea and Secondary Dysmenorrhea), Treatment (Hormonal Therapy, Pain Reliever, Surgery and Others), End-Users (Hospitals, Homecare, Speciality Centres, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Amgen Inc. (U.S.), Boehringer Ingelheim International GmbH. (Germany)., Ipsen Pharma (France), Spectrum Pharmaceuticals, Inc. (U.S.), Takeda Pharmaceutical Company Limited. (Japan)., BeyondSpring Inc. (U.S.), Apotex Inc. (Canada), Taj Pharmaceuticals, Limited. (India), Sanofi (France), Terramedic Incorporated (Philippines), Nua (India), Cora (India) |
|
Market Opportunities |
|
Market Definition
Dysmenorrhea is a type of condition that is characterized by painful periods and menstrual cramps. The condition occurs with unbearable pain occurs during menstruation in females. Symptoms typically last less than three days; the pain is generally in the pelvis or lower abdomen. The pain typically occurs 1 or 2 days before the menstrual bleeding starts and is usually felt in the lower abdomen area, thighs and back. The severity of the pain could vary and can also be stayed for 12 to 72 hours. The most common symptoms of dysmenorrhea are fatigue, diarrhoea, mood swings.
Global Dysmenorrhea Treatment Market Dynamics
Drivers
- Increasing Symptoms of Period Pain
As per the article published in the Women's Health Concern and British Menopause Society in December 2020, around 80% of dysmenorrhea women experience period pain at some stage in their lifetime. In about 40% of women, period pain is accompanied by premenstrual symptoms, such as bloating, swollen stomach, tender breasts, lack of concentration, mood swings, clumsiness, and tiredness. This boosts the market growth.
- Growing Research Studies About The Disease
According to the study titled "Fixed-Dose Combination of NSAIDs and Spasmolytic Agents in the Treatment of Different Types of Pain - A Practical Review", published in July 2021, many combinations of non-steroidal anti-inflammatory drugs (NSAIDs) and spasmolytics have been developed and tested, indicating a huge potential for future research to develop modern, effective combinations and search for new indications for the existing fixed-dose combination drugs for the treatment of dysmenorrhea.
Opportunities
- Increasing Launch of New Products
The launch of numerous new products for treating pain related with primary and secondary dysmenorrhea is also boosting the studied market. For instance, in September 2021, Myovant Sciences and Pfizer Inc. recieved the approval from U.S. FDA for the review of a supplemental New Drug Application (sNDA) for MYFEMBREE (relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) to manage moderate to severe pain associated with endometriosis. Thus, these several launches create much market opportunities.
- Rising Start-up Launches For Reproductive Health
Several start-ups have been launched by various organizations, leading to market growth. For instance, Gynica is a FemTech startup based in Israel. It is mainly responsible for the manufacturing of cannabis-based treatments for women's reproductive health. Gynica is a medical firm which is focused on studying and developing marijuana treatments for several gynecological disorders and symptoms. They are members of Station F's FemTech Cohort. This creates more opportunity in the market.
Restraints/Challenges
The high cost of care will hinder the market's growth rate during the forecast period of 2022-2030. Lack of public awareness and inadequate reimbursement policies may impede and impede market expansion during the projection period of 2022-2030.
Moreover, challenges related with long-term care and other side effects and issues, the high morbidity of menstrual cramps because of insufficient treatments, and traditional family values are projected to decrease the growth of the dysmenorrhea treatment market.
This global dysmenorrhea treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global dysmenorrhea treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Global Dysmenorrhea Treatment Market
COVID-19 has impacted the emotional and mental health of the people majorly. It has been witnessed that periods of stress and psychological distress can affect a woman’s menstrual health. The severe changes in menstrual cycles can lead to higher episodes of dysmenorrhea, considerably impacting the market. As per the study titled "The Impact of the COVID-19 Pandemic on Women’s Reproductive Health", published in March 2021, 46% of women reported a change in their menstrual cycle, with 30% new dysmenorrhea cases, since the beginning of the pandemic. Thus, COVID-19 has impacted the dysmenorrhea treatment market significantly.
Recent Development:
- In December 2020, Nua announced the release of a first-of-its-kind period pain relief solution called Cramps Comfort. Nua has released a revolutionary self-heating patch that can deliver up to 8 hours of heat to improve period discomfort.
- In 2022, Cora® debuted its fresh comfort-focused brand image, feel, and identity. Furthermore, company has expanded its range of products to fulfil the need of consumers.
Global Dysmenorrhea Treatment Market Scope
The global dysmenorrhea treatment market is segmented on the basis of type, treatment, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Primary Dysmenorrhea
- Secondary Dysmenorrhea
Treatment
- Hormonal Therapy
- Pain Reliever
- Surgery
- Others
End-Users
- Hospitals
- Homecare
- Speciality Centres
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Dysmenorrhea Treatment Market Regional Analysis/Insights
The dysmenorrhea treatment market is analyzed and market size insights and trends are provided by type, treatment, distribution channel and end-user as referenced above.
The major countries covered in the dysmenorrhea treatment market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is expected to have the highest market growth due to the presence of key product manufacturers and increasing research and development activities.
Asia-Pacific dominates the market due to increased new research and developments on dysmenorrhea treatment market.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Dysmenorrhea Treatment Market Share Analysis
The dysmenorrhea treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to dysmenorrhea treatment market.
Key players operating in the dysmenorrhea treatment market include:
- Amgen Inc. (U.S.)
- Boehringer Ingelheim International GmbH. (Germany)
- Ipsen Pharma (France)
- Spectrum Pharmaceuticals, Inc. (U.S.)
- Takeda Pharmaceutical Company Limited. (Japan)
- BeyondSpring Inc. (U.S.)
- Apotex Inc. (Canada)
- Taj Pharmaceuticals, Limited. (India)
- Sanofi (France)
- Terramedic Incorporated (Philippines)
- Nua (India)
- Cora (India)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL DYSMENORRHEA TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL DYSMENORRHEA TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 SALES VOLUME DATA
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL DYSMENORRHEA TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
5.3 COMPETITIVE INTELLIGENCE
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.1.1 PATENT LANDSCAPE
6.1.2 USPTO NUMBER
6.1.3 PATENT EXPIRY
6.1.4 EPIO NUMBER
6.1.5 PATENT STRENGTH AND QUALITY
6.1.6 PATENT CLAIMS
6.1.7 PATENT CITATIONS
6.1.8 PATENT LITIGATION AND LICENSING
6.1.9 FILE OF PATENT
6.1.10 PATENT RECEIVED CONTRIES
6.1.11 TECHNOLOGY BACKGROUND
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH SPECIALIST
6.8 OTHER KOL SNAPSHOTS
7 EPIDEMIOLOGY
7.1 INCIDENCE OF ALL BY GENDER
7.2 TREATMENT RATE
7.3 MORTALITY RATE
7.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
7.5 PATIENT TREATMENT SUCCESS RATES
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 REGULATORY FRAMEWORK
9.1 REGULATORY APPROVAL PROCESS
9.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
9.3 REGULATORY APPROVAL PATHWAYS
9.4 LICENSING AND REGISTRATION
9.5 POST-MARKETING SURVEILLANCE
9.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
10 PIPELINE ANALYSIS
10.1 CLINICAL TRIALS AND PHASE ANALYSIS
10.2 DRUG THERAPY PIPELINE
10.3 PHASE III CANDIDATES
10.4 PHASE II CANDIDATES
10.5 PHASE I CANDIDATES
10.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 CLINICAL TRIALS FOR DYSMENORRHEA TREATMENT MARKET
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE: DYSMENORRHEA TREATMENT MARKET
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE: DYSMENORRHEA TREATMENT MARKET
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE DYSMENORRHEA TREATMENT MARKET
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR DYSMENORRHEA TREATMENT MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
11 MARKETED DRUG ANALYSIS
11.1 DRUG
11.1.1 BRAND NAME
11.1.2 GENERICS NAME
11.2 THERAPEUTIC INDIACTION
11.3 PHARACOLOGICAL CLASS OD THE DRUG
11.4 DRUG PRIMARY INDICATION
11.5 MARKET STATUS
11.6 MEDICATION TYPE
11.7 DRUG DOSAGES FORM
11.8 DOSAGES AVAILABILITY
11.9 PACKAGING TYPE
11.1 DRUG ROUTE OF ADMINISTRATION
11.11 DOSING FREQUENCY
11.12 DRUG INSIGHT
11.13 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
11.13.1 FORECAST MARKET OUTLOOK
11.13.2 CROSS COMPETITION
11.13.3 THERAPEUTIC PORTFOLIO
11.13.4 CURRENT DEVELOPMENT SCENARIO
12 MARKET ACCESS
12.1 10-YEAR MARKET FORECAST
12.2 CLINICAL TRIAL RECENT UPDATES
12.3 ANNUAL NEW FDA APPROVED DRUGS
12.4 DRUGS MANUFACTURER AND DEALS
12.5 MAJOR DRUG UPTAKE
12.6 CURRENT TREATMENT PRACTICES
12.7 IMPACT OF UPCOMING THERAPY
13 R & D ANALYSIS
13.1 COMPARATIVE ANALYSIS
13.2 DRUG DEVELOPMENTAL LANDSCAPE
13.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
13.4 THERAPEUTIC ASSESSMENT
13.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
14 MARKET OVERVIEW
14.1 DRIVERS
14.2 RESTRAINTS
14.3 OPPORTUNITIES
14.4 CHALLENGES
15 GLOBAL DYSMENORRHEA TREATMENT MARKET, BY TYPE
15.1 OVERVIEW
15.1.1 PRIMARY DYSMENORRHEA
15.1.2 SECONDARY DYSMENORRHEA
15.1.2.1. ENDOMETRIOSIS-RELATED DYSMENORRHEA
15.1.2.2. UTERINE FIBROIDS-RELATED DYSMENORRHEA
15.1.2.3. PELVIC INFLAMMATORY DISEASE (PID) DYSMENORRHEA
15.1.2.4. ADENOMYOSIS-RELATED DYSMENORRHEA
15.1.2.5. CERVICAL STENOSIS-RELATED DYSMENORRHEA
15.1.2.6. OTHERS
16 GLOBAL DYSMENORRHEA TREATMENT MARKET, BY TREATMENT TYPE
16.1 OVERVIEW
16.2 PAIN RELIEVERS
16.2.1 NONSTEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS)
16.2.1.1. IBUPROFEN
16.2.1.2. NAPROXEN
16.2.1.3. MEFENAMIC ACID
16.2.1.4. DICLOFENAC
16.2.1.5. ASPIRIN
16.2.2 ANALGESICS & MUSCLE RELAXANTS
16.2.2.1. ACETAMINOPHEN
16.2.2.2. OPIOIDS
16.2.2.3. CODEINE
16.2.2.4. TRAMADOL
16.2.2.5. OXYCODONE
16.2.2.6. OTHERS
16.2.3 CYCLOBENZAPRINE & BACLOFEN
16.2.4 OTHERS
16.3 HORMONAL THERAPY
16.3.1 COMBINED ORAL CONTRACEPTIVES (MONOPHASIC OR MULTIPHASIC)
16.3.1.1. NORGESTIMATE/ETHINYL ESTRADIOL
16.3.1.2. NORETHINDRONE/ETHINYL ESTRADIOL
16.3.2 EXTENDED-CYCLE ORAL CONTRACEPTIVES
16.3.2.1. LEVONORGESTREL/ETHINYL ESTRADIOL
16.3.2.2. LEVONORGESTREL/ETHINYL ESTRADIOL
16.3.2.3. OTHER
16.3.3 ETONOGESTREL IMPLANT
16.3.4 ETONOGESTREL/ETHINYL ESTRADIOL
16.3.5 LEVONORGESTREL-RELEASING INTRAUTERINE SYSTEM
16.3.6 MEDROXYPROGESTERONE SHOT
16.4 SURGICAL TREATMENT
16.4.1 MINIMALLY INVASIVE SURGERIES
16.4.1.1. LAPAROSCOPIC SURGERY (FOR ENDOMETRIOSIS, FIBROIDS)
16.4.1.2. HYSTEROSCOPY (FOR UTERINE ABNORMALITIES)
16.4.2 MAJOR SURGICAL PROCEDURES
16.4.2.1. HYSTERECTOMY (UTERUS REMOVAL)
16.4.2.2. MYOMECTOMY (FIBROID REMOVAL)
16.4.2.3. ENDOMETRIAL ABLATION
16.4.2.4. OTHER
16.5 OTHER THERAPY
16.5.1 HEAT THERAPY
16.5.1.1. PATCH
16.5.1.2. WRAP
16.5.1.3. CERAMIC BELT EMITTING FAR-INFRARED RADIATION (FIR)
16.5.2 BEHAVIORAL COUNSELING
16.5.2.1. DESENSITIZATION-BASED PROCEDURES
16.5.2.2. HYPNOTHERAPY
16.5.2.3. IMAGERY
16.5.2.4. COPING STRATEGIES
16.5.2.5. OTHERS
16.5.3 DIET AND VITAMINS
16.5.3.1. VITAMIN E
16.5.3.2. VITAMIN B
16.5.3.3. VITAMIN D
16.5.3.4. OTHERS
16.6 OTHERS
17 GLOBAL DYSMENORRHEA TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
17.1 OVERVIEW
17.2 ORAL
17.3 PARENTERAL
17.4 TOPICAL
17.5 OTHERS
18 GLOBAL DYSMENORRHEA TREATMENT MARKET, BY DRUG TYPE
18.1 OVERVIEW
18.2 BRANDED
18.3 GENERIC
19 GLOBAL DYSMENORRHEA TREATMENT MARKET, BY MODE OF PURCHASE
19.1 OVERVIEW
19.2 PRESCRIPTION BASED
19.3 OVER THE COUNTER
20 GLOBAL DYSMENORRHEA TREATMENT MARKET, BY END USER
20.1 OVERVIEW
20.2 HOSPITALS
20.3 AMUBALATORY SURGICAL CENTERS
20.4 SPECIALTY CLINICS
20.5 RESEARCH & ACADEMIC INSTITUTES
20.6 HOME HEALTHCARE
20.7 OTHERS
21 GLOBAL DYSMENORRHEA TREATMENT MARKET, BY DISTRIBUTION CHANNEL
21.1 OVERVIEW
21.2 DIRECT TENDER
21.3 RETAIL SALES
21.3.1 ONLINE
21.3.2 OFFLINE
21.4 OTHERS
22 GLOBAL DYSMENORRHEA TREATMENT MARKET, BY GEOGRAPHY
22.1 GLOBAL DYSMENORRHEA TREATMENT MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
22.2 NORTH AMERICA
22.2.1 U.S.
22.2.2 CANADA
22.2.3 MEXICO
22.3 EUROPE
22.3.1 GERMANY
22.3.2 FRANCE
22.3.3 U.K.
22.3.4 ITALY
22.3.5 SPAIN
22.3.6 RUSSIA
22.3.7 TURKEY
22.3.8 BELGIUM
22.3.9 NETHERLANDS
22.3.10 SWITZERLAND
22.3.11 REST OF EUROPE
22.4 ASIA PACIFIC
22.4.1 JAPAN
22.4.2 CHINA
22.4.3 SOUTH KOREA
22.4.4 INDIA
22.4.5 AUSTRALIA
22.4.6 SINGAPORE
22.4.7 THAILAND
22.4.8 MALAYSIA
22.4.9 INDONESIA
22.4.10 PHILIPPINES
22.4.11 REST OF ASIA PACIFIC
22.5 SOUTH AMERICA
22.5.1 BRAZIL
22.5.2 ARGENTINA
22.5.3 REST OF SOUTH AMERICA
22.6 MIDDLE EAST AND AFRICA
22.6.1 SOUTH AFRICA
22.6.2 EGYPT
22.6.3 SAUDI ARABIA
22.6.4 U.A.E
22.6.5 ISRAEL
22.6.6 REST OF MIDDLE EAST AND AFRICA
22.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
23 GLOBAL DYSMENORRHEA TREATMENT MARKET,COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: GLOBAL
23.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
23.3 COMPANY SHARE ANALYSIS: EUROPE
23.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
23.5 MERGERS & ACQUISITIONS
23.6 NEW PRODUCT DEVELOPMENT AND APPROVALS
23.7 EXPANSIONS
23.8 REGULATORY CHANGES
23.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 GLOBAL DYSMENORRHEA TREATMENT MARKET , SWOT & DBMR ANALYSIS
25 GLOBAL DYSMENORRHEA TREATMENT MARKET, COMPANY PROFILE
25.1 GLAXOSMITHKLINE PLC.
25.1.1 COMPANY OVERVIEW
25.1.2 REVENUE ANALYSIS
25.1.3 GEOGRAPHIC PRESENCE
25.1.4 PRODUCT PORTFOLIO
25.1.5 RECENT DEVELOPMENTS
25.2 CUMBERLAND PHARMACEUTICALS INC
25.2.1 COMPANY OVERVIEW
25.2.2 REVENUE ANALYSIS
25.2.3 GEOGRAPHIC PRESENCE
25.2.4 PRODUCT PORTFOLIO
25.2.5 RECENT DEVELOPMENTS
25.3 BAYER AG
25.3.1 COMPANY OVERVIEW
25.3.2 REVENUE ANALYSIS
25.3.3 GEOGRAPHIC PRESENCE
25.3.4 PRODUCT PORTFOLIO
25.3.5 RECENT DEVELOPMENTS
25.4 PFIZER INC.
25.4.1 COMPANY OVERVIEW
25.4.2 REVENUE ANALYSIS
25.4.3 GEOGRAPHIC PRESENCE
25.4.4 PRODUCT PORTFOLIO
25.4.5 RECENT DEVELOPMENTS
25.5 MYLAN N.V.
25.5.1 COMPANY OVERVIEW
25.5.2 REVENUE ANALYSIS
25.5.3 GEOGRAPHIC PRESENCE
25.5.4 PRODUCT PORTFOLIO
25.5.5 RECENT DEVELOPMENTS
25.6 ALVOGEN
25.6.1 COMPANY OVERVIEW
25.6.2 REVENUE ANALYSIS
25.6.3 GEOGRAPHIC PRESENCE
25.6.4 PRODUCT PORTFOLIO
25.6.5 RECENT DEVELOPMENTS
25.7 MERCK & CO., INC
25.7.1 COMPANY OVERVIEW
25.7.2 REVENUE ANALYSIS
25.7.3 GEOGRAPHIC PRESENCE
25.7.4 PRODUCT PORTFOLIO
25.7.5 RECENT DEVELOPMENTS
25.8 TEVA PHARMACEUTICAL INDUSTRIES LTD.
25.8.1 COMPANY OVERVIEW
25.8.2 REVENUE ANALYSIS
25.8.3 GEOGRAPHIC PRESENCE
25.8.4 PRODUCT PORTFOLIO
25.8.5 RECENT DEVELOPMENTS
25.9 BOEHRINGER INGELHEIM
25.9.1 COMPANY OVERVIEW
25.9.2 REVENUE ANALYSIS
25.9.3 GEOGRAPHIC PRESENCE
25.9.4 PRODUCT PORTFOLIO
25.9.5 RECENT DEVELOPMENTS
25.1 LUPIN
25.10.1 COMPANY OVERVIEW
25.10.2 REVENUE ANALYSIS
25.10.3 GEOGRAPHIC PRESENCE
25.10.4 PRODUCT PORTFOLIO
25.10.5 RECENT DEVELOPMENTS
25.11 SANOFI
25.11.1 COMPANY OVERVIEW
25.11.2 REVENUE ANALYSIS
25.11.3 GEOGRAPHIC PRESENCE
25.11.4 PRODUCT PORTFOLIO
25.11.5 RECENT DEVELOPMENTS
25.12 COMFORTÉ
25.12.1 COMPANY OVERVIEW
25.12.2 REVENUE ANALYSIS
25.12.3 GEOGRAPHIC PRESENCE
25.12.4 PRODUCT PORTFOLIO
25.12.5 RECENT DEVELOPMENTS
25.13 BIOELECTRONICS CORPORATION
25.13.1 COMPANY OVERVIEW
25.13.2 REVENUE ANALYSIS
25.13.3 GEOGRAPHIC PRESENCE
25.13.4 PRODUCT PORTFOLIO
25.13.5 RECENT DEVELOPMENTS
25.14 ABBVIE INC.
25.14.1 COMPANY OVERVIEW
25.14.2 REVENUE ANALYSIS
25.14.3 GEOGRAPHIC PRESENCE
25.14.4 PRODUCT PORTFOLIO
25.14.5 RECENT DEVELOPMENTS
25.15 NOBELPHARMA AMERICA, LLC.
25.15.1 COMPANY OVERVIEW
25.15.2 REVENUE ANALYSIS
25.15.3 GEOGRAPHIC PRESENCE
25.15.4 PRODUCT PORTFOLIO
25.15.5 RECENT DEVELOPMENTS
25.16 MYOVANT SCIENCES GMBH
25.16.1 COMPANY OVERVIEW
25.16.2 REVENUE ANALYSIS
25.16.3 GEOGRAPHIC PRESENCE
25.16.4 PRODUCT PORTFOLIO
25.16.5 RECENT DEVELOPMENTS
25.17 NOVARTIS AG
25.17.1 COMPANY OVERVIEW
25.17.2 REVENUE ANALYSIS
25.17.3 GEOGRAPHIC PRESENCE
25.17.4 PRODUCT PORTFOLIO
25.17.5 RECENT DEVELOPMENTS
25.18 TEVA PHARMACEUTICAL INDUSTRIES LTD.
25.18.1 COMPANY OVERVIEW
25.18.2 REVENUE ANALYSIS
25.18.3 GEOGRAPHIC PRESENCE
25.18.4 PRODUCT PORTFOLIO
25.18.5 RECENT DEVELOPMENTS
25.19 AMERICAN REGENT, INC. (A SUBSIDIARY OF DAIICHI SANKYO GROUP COMPANY)
25.19.1 COMPANY OVERVIEW
25.19.2 REVENUE ANALYSIS
25.19.3 GEOGRAPHIC PRESENCE
25.19.4 PRODUCT PORTFOLIO
25.19.5 RECENT DEVELOPMENTS
25.2 OBSEVA
25.20.1 COMPANY OVERVIEW
25.20.2 REVENUE ANALYSIS
25.20.3 GEOGRAPHIC PRESENCE
25.20.4 PRODUCT PORTFOLIO
25.20.5 RECENT DEVELOPMENTS
25.21 NEUROCRINE BIOSCIENCES
25.21.1 COMPANY OVERVIEW
25.21.2 REVENUE ANALYSIS
25.21.3 GEOGRAPHIC PRESENCE
25.21.4 PRODUCT PORTFOLIO
25.21.5 RECENT DEVELOPMENTS
26 CONCLUSION
27 QUESTIONNAIRE
28 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












