Global Edaravone Market
Market Size in USD Billion
CAGR :
% 
 USD
1.60 Billion
USD
2.80 Billion
2024
2032
USD
1.60 Billion
USD
2.80 Billion
2024
2032
| 2025 –2032 | |
| USD 1.60 Billion | |
| USD 2.80 Billion | |
|
|
|
|
Edaravone Market Size
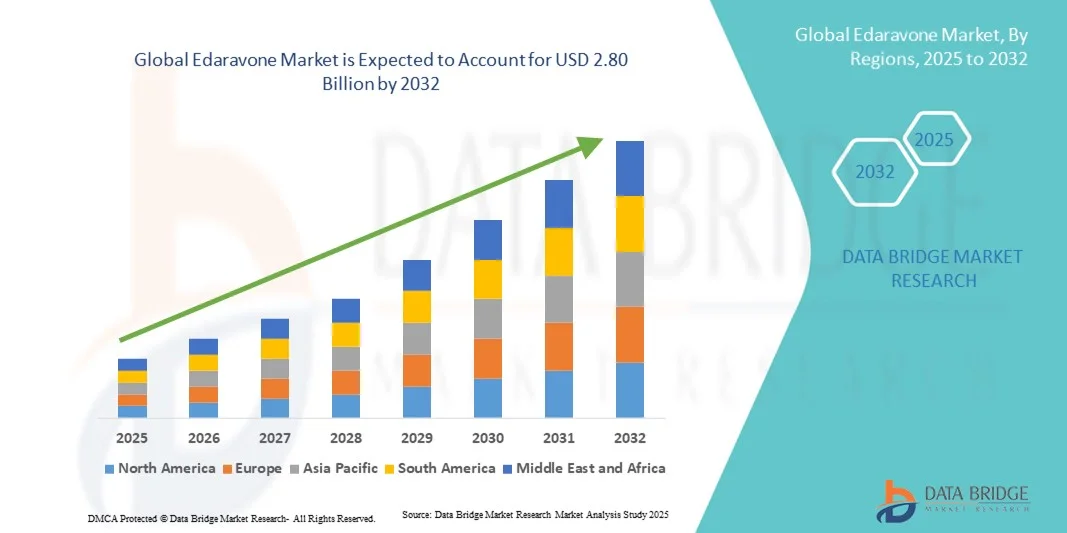
- The global Edaravone market size was valued at USD 1.60 billion in 2024 and is expected to reach USD 2.80 billion by 2032, at a CAGR of 7.2% during the forecast period
- The market growth is primarily driven by the increasing prevalence of neurological disorders such as amyotrophic lateral sclerosis (ALS) and ischemic stroke, conditions for which Edaravone has demonstrated efficacy in slowing disease progression
- The growing demand for effective treatments for neurodegenerative diseases is establishing Edaravone as a key player in the global pharmaceutical market, thereby significantly boosting the industry's growth. Advancements in medical research and technology, along with rising awareness about Edaravone's therapeutic benefits, are further accelerating its adoption in clinical settings
Edaravone Market Analysis
- Edaravones, a neuroprotective drug primarily used in the treatment of amyotrophic lateral sclerosis (ALS) and acute ischemic stroke, is increasingly vital in modern neurological care due to its efficacy in slowing disease progression, reducing oxidative stress, and improving patient outcomes in both hospital and home-care settings
- The escalating demand for Edaravone is primarily fueled by the rising prevalence of neurodegenerative diseases, growing awareness among patients and healthcare providers, and ongoing advancements in clinical research supporting its therapeutic benefits
- North America dominated the Edaravone market with the largest revenue share of 40.5% in 2024, characterized by advanced healthcare infrastructure, high healthcare spending, and strong presence of key pharmaceutical players, with the U.S. experiencing substantial adoption of Edaravone therapies, particularly in hospitals and specialized neurological centers, driven by innovations in formulation and delivery methods
- Asia-Pacific is expected to be the fastest-growing region in the Edaravone market during the forecast period due to improving healthcare facilities, increasing healthcare awareness, and a growing elderly population prone to neurodegenerative disorders
- Injection segment dominated the Edaravone market in 2024, with a market share of 65.3%, driven by their established therapeutic efficacy, ease of administration in clinical settings, and preference among healthcare professionals for acute treatment protocols
Report Scope and Edaravone Market Segmentation
|
Attributes |
Edaravone Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Edaravone Market Trends
Rising Adoption of Neuroprotective Therapies in ALS and Stroke Care
- A significant and accelerating trend in the global Edaravone market is the growing use of neuroprotective therapies for the management of ALS and acute ischemic stroke, enhancing patient outcomes and slowing disease progression
- For instance, Edaravone IV infusion therapy is increasingly administered in hospitals and specialized neurological care centers, allowing clinicians to manage oxidative stress and motor function deterioration effectively
- Integration of Edaravone treatment protocols with advanced patient monitoring and hospital management systems enables personalized dosing, improved treatment adherence, and intelligent alerts for therapy response, improving overall clinical outcomes
- The combination of Edaravone with other neurorehabilitation strategies facilitates a holistic care approach, enabling patients and caregivers to manage disease progression, monitor therapy, and optimize recovery simultaneously
- This trend towards more precise, technology-assisted neuroprotective care is reshaping patient expectations and clinical practice, with pharmaceutical companies developing streamlined infusion protocols and supportive tools
- The demand for Edaravone therapies that offer clinically validated neuroprotective benefits is growing rapidly across hospital and outpatient sectors, as physicians increasingly prioritize evidence-based management of neurological disorders
Edaravone Market Dynamics
Driver
Increasing Prevalence of Neurodegenerative Disorders
- The rising incidence of ALS, ischemic stroke, and related neurological disorders is a significant driver for the increasing adoption of Edaravone in hospitals and specialized clinics
- For instance, in March 2024, a leading pharmaceutical company reported expanded hospital access programs for Edaravone, aiming to improve therapy reach in high-incidence regions, expected to drive market growth
- As patients and caregivers seek effective therapies to slow disease progression and improve quality of life, Edaravone offers clinically proven neuroprotective effects and improved motor function outcomes
- Furthermore, increasing awareness among neurologists and healthcare institutions regarding the benefits of Edaravone integration in acute and chronic care settings is expanding its clinical adoption
- Growing investments in neurology-focused research and hospital infrastructure are enabling wider Edaravone accessibility, particularly in urban medical centers
- Regulatory approvals in new regions and faster market authorizations are driving adoption, as clinicians can more readily prescribe Edaravone for eligible patients
- The convenience of hospital-administered infusion protocols, patient monitoring systems, and supportive treatment regimens is propelling the use of Edaravone in both developed and emerging markets
Restraint/Challenge
High Cost and Limited Awareness in Emerging Regions
- The relatively high cost of Edaravone therapy, particularly in emerging markets, poses a significant challenge to broader adoption, limiting accessibility for patients with budget constraints
- For instance, patients in regions with limited insurance coverage or healthcare infrastructure may not afford regular infusion therapy, restricting widespread clinical uptake
- Addressing affordability through patient assistance programs, generic formulations, and cost-effective distribution is critical to overcoming market entry barriers and expanding reach
- Moreover, limited awareness among patients and healthcare providers about the therapeutic benefits of Edaravone restricts demand, especially outside specialized neurology centers
- Concerns regarding side effects such as renal impairment or liver function changes may limit prescribing by cautious healthcare providers, posing a challenge for wider adoption
- Complex administration protocols and the need for hospital-based infusion sessions can be inconvenient for patients, acting as a barrier to frequent or long-term therapy adherence
- Overcoming these challenges through educational initiatives, government support programs, and broader market access strategies will be vital for sustained Edaravone market growth
Edaravone Market Scope
The market is segmented on the basis of product, end users, and distribution channels.
- By Product
On the basis of product, the Edaravone market is segmented into injection and oral formulations. The Injection segment dominated the market with the largest market revenue share of 65.3% in 2024, driven by its established therapeutic efficacy and preference in clinical settings. Hospitals and specialty clinics often prioritize injection formulations for their rapid onset of action and precise dosing capabilities. The segment benefits from extensive physician familiarity and approval, particularly in ALS and ischemic stroke management. The availability of standardized infusion protocols and hospital-administered treatments further strengthens its adoption. In addition, injectable Edaravone is supported by clinical evidence demonstrating better neuroprotective outcomes, making it the preferred choice in acute care settings. Growing hospital infrastructure and trained healthcare professionals also reinforce the dominance of this segment.
The Oral segment is expected to witness the fastest growth rate of 12.5% from 2025 to 2032, fueled by increasing patient preference for home-based and outpatient therapies. Oral formulations offer convenience and reduce the need for hospital visits, making them suitable for long-term therapy adherence. Pharmaceutical companies are investing in bioavailable oral versions to expand accessibility and patient compliance. The segment is particularly attractive in emerging markets with limited hospital infrastructure. Furthermore, ongoing research on oral Edaravone aims to optimize dosing and efficacy comparable to injections. As awareness of oral therapy benefits increases, this segment is anticipated to grow rapidly across home healthcare and outpatient settings.
- By End Users
On the basis of end users, the Edaravone market is segmented into hospitals, clinics, specialty clinics, home healthcare, and others. The Hospitals segment dominated the market in 2024, accounting for the largest revenue share due to the concentration of neurological specialists and infrastructure for infusion therapies. Hospitals provide controlled environments for administering Edaravone injections, ensuring accurate dosing and patient monitoring. The segment benefits from high patient volumes, especially in urban centers with ALS and stroke prevalence. Hospitals also play a crucial role in clinical studies, further validating Edaravone’s efficacy. Integration of patient monitoring systems and electronic health records enhances therapy management in hospitals. In addition, hospitals are preferred for acute stroke treatment, reinforcing their dominance in the market.
The Home Healthcare segment is expected to witness the fastest growth rate of 13.2% from 2025 to 2032, driven by the rising preference for home-based treatment and patient convenience. Patients with chronic ALS or mobility challenges increasingly prefer therapy at home to avoid frequent hospital visits. Oral and portable infusion options facilitate the expansion of home healthcare services. Telemedicine support and nurse-assisted infusion programs also contribute to adoption. Growing awareness of Edaravone’s benefits among caregivers and patients further boosts market potential. The segment’s flexibility and comfort make it a key driver of future growth in the Edaravone market.
- By Distribution Channel
On the basis of distribution channel, the Edaravone market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. The Hospital Pharmacies segment dominated the market in 2024, driven by direct availability of Edaravone for inpatient and outpatient treatments in healthcare facilities. Hospitals ensure storage, administration, and adherence to clinical protocols, enhancing therapy safety and effectiveness. The segment benefits from physician prescriptions and hospital-managed insurance coverage. Strong hospital networks in developed regions support large-scale adoption. In addition, hospital pharmacies play a critical role in patient education and monitoring during therapy. The preference for injectable formulations further reinforces hospital pharmacies as the primary distribution channel.
The Online Pharmacies segment is expected to witness the fastest growth rate of 14.1% from 2025 to 2032, fueled by increasing e-commerce adoption and home delivery of specialty drugs. Patients prefer ordering Edaravone online for convenience, especially in regions where local pharmacies have limited stock. Digital platforms also offer subscription services, reminders, and telehealth integration. Growing trust in online pharmacies and expanding internet penetration support this trend. Online distribution reduces geographical barriers and provides access to remote patients. As oral and home-use formulations gain popularity, online pharmacies are expected to become a major channel for Edaravone.
Edaravone Market Regional Analysis
- North America dominated the Edaravone market with the largest revenue share of 40.5% in 2024, characterized by advanced healthcare infrastructure, high healthcare spending, and strong presence of key pharmaceutical players, with the U.S. experiencing substantial adoption of Edaravone therapies, particularly in hospitals and specialized neurological centers, driven by innovations in formulation and delivery methods
- Patients and healthcare providers in the region highly value the proven clinical efficacy, rapid therapeutic action, and hospital-administered protocols offered by Edaravone injection therapy, ensuring accurate dosing and effective patient monitoring
- This widespread adoption is further supported by strong R&D investments, high healthcare spending, and the presence of key pharmaceutical players actively expanding Edaravone access, establishing it as a preferred neuroprotective therapy in hospitals and specialty clinics across the U.S. and Canada
U.S. Edaravone Market Insight
The U.S. Edaravone market captured the largest revenue share of 81% in 2024 within North America, fueled by the high prevalence of ALS and ischemic stroke, coupled with advanced healthcare infrastructure. Hospitals and specialty clinics prioritize Edaravone infusion therapy for its proven neuroprotective benefits and precise dosing protocols. The growing emphasis on early intervention and improved patient outcomes drives adoption, alongside strong R&D investments and robust reimbursement frameworks. Moreover, telemedicine support and home healthcare initiatives are further expanding access, significantly contributing to market growth.
Europe Edaravone Market Insight
The Europe Edaravone market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing awareness of neurological disorders and government support for rare disease treatments. The rise in hospital-based neurorehabilitation programs, coupled with the demand for effective neuroprotective therapies, is fostering Edaravone adoption. European patients and healthcare providers are drawn to its clinical efficacy and integration with patient monitoring systems. The region is experiencing significant growth across hospitals, specialty clinics, and home healthcare settings, with Edaravone being incorporated into both acute and long-term care protocols.
U.K. Edaravone Market Insight
The U.K. Edaravone market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the increasing focus on neurological care and patient quality of life. Rising incidence of ALS and ischemic stroke encourages hospitals and clinics to adopt Edaravone therapies. In addition, awareness campaigns and educational initiatives targeting neurologists and caregivers are supporting therapy uptake. The U.K.’s well-developed healthcare infrastructure and strong insurance coverage facilitate broader access, while integration with outpatient and home healthcare programs is expected to continue to stimulate market growth.
Germany Edaravone Market Insight
The Germany Edaravone market is expected to expand at a considerable CAGR during the forecast period, fueled by growing awareness of neuroprotective therapies and the demand for technologically advanced hospital treatments. Germany’s well-developed healthcare system and emphasis on evidence-based medicine promote Edaravone adoption in both hospitals and specialty clinics. Integration of Edaravone with patient monitoring and electronic health systems is increasingly prevalent, with a strong focus on optimizing dosing protocols and improving patient outcomes. Sustainable healthcare initiatives also support adoption in outpatient and home healthcare programs.
Asia-Pacific Edaravone Market Insight
The Asia-Pacific Edaravone market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032, driven by increasing prevalence of neurodegenerative disorders, improving healthcare infrastructure, and rising awareness in countries such as Japan, China, and India. The region’s focus on expanding hospital networks and home healthcare services is driving therapy adoption. Furthermore, telemedicine, remote monitoring, and training programs for caregivers are enhancing access. As APAC countries invest in neurological care and supportive infrastructure, affordability and availability of Edaravone therapies are expanding to a wider patient base.
Japan Edaravone Market Insight
The Japan Edaravone market is gaining momentum due to the country’s high prevalence of ALS and acute stroke cases, combined with a strong focus on advanced medical treatments. Hospitals and specialty clinics prioritize Edaravone infusion therapy for its neuroprotective effects, supported by clinical evidence and established treatment protocols. The integration of therapy with patient monitoring systems and rehabilitation programs is fueling growth. Moreover, Japan’s aging population is expected to increase demand for hospital-administered and home-based Edaravone solutions in both residential and clinical settings.
India Edaravone Market Insight
The India Edaravone market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to the increasing prevalence of neurological disorders, improving healthcare infrastructure, and expanding access to hospitals and specialty clinics. The country’s growing middle class and increasing healthcare awareness are driving demand for neuroprotective therapies. Initiatives promoting outpatient and home healthcare services, combined with the availability of affordable Edaravone formulations, are key factors propelling market growth. Strong government support for rare disease treatment programs further enhances therapy adoption in India.
Edaravone Market Share
The Edaravone industry is primarily led by well-established companies, including:
- Teva Pharmaceutical Industries Ltd. (Israel)
- Biogen Inc. (U.S.)
- Viatris Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Hikma Pharmaceuticals PLC (U.K.)
- Dr. Reddy’s Laboratories Ltd. (India)
- Cipla Ltd. (India)
- Lupin (U.S.)
- Zydus Lifesciences Ltd. (India)
- Amgen Inc. (U.S.)
- Novartis AG (Switzerland)
- Sanofi (France)
- Boehringer Ingelheim International GmbH (Germany)
- Eisai Co., Ltd. (Japan)
- Otsuka Pharmaceutical Co., Ltd. (Japan)
- Takeda Pharmaceutical Company Limited (Japan)
- Astellas Pharma Inc. (Japan)
- Bristol-Myers Squibb Company (U.S.)
- AbbVie Inc. (U.S.)
What are the Recent Developments in Global Edaravone Market?
- In August 2025, Mitsubishi Tanabe Pharma America announced the publication of a long-term function and survival analysis of RADICAVA ORS (edaravone)-treated patients with ALS. The study demonstrated sustained benefits in functional outcomes and survival, reinforcing the efficacy of the oral formulation in managing ALS
- In May 2025, Mitsubishi Tanabe Pharma Corporation announced the launch of RADICAVA (edaravone) for the treatment of amyotrophic lateral sclerosis (ALS) in Australia. This intravenous formulation became available through a local licensee, Teva Pharma Australia Pty Ltd, expanding treatment options for ALS patients in the region
- In October 2024, Mitsubishi Tanabe Pharma America, Inc. announced the strategic decision to discontinue intravenous (IV) RADICAVA (edaravone) in the U.S. This decision was not due to safety or effectiveness concerns but was made to streamline treatment options and focus on the oral formulation
- In October 2024, Mitsubishi Tanabe Pharma America presented new real-world data at the Academy of Managed Care Pharmacy (AMCP) Nexus 2024 meeting. The data, derived from a U.S.-based administrative claims database, highlighted healthcare resource utilization among ALS patients treated with RADICAVA ORS®, providing valuable insights into the treatment's real-world impact
- In April 2024, the U.S. Food and Drug Administration (FDA) granted 7-year orphan drug exclusivity to RADICAVA ORS (edaravone). This designation recognizes the oral formulation's major contribution to patient care by providing an alternative to the intravenous route of administration for ALS treatment
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.