Global Electro Medical Devices In Alzheimers Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
4.99 Billion
USD
10.66 Billion
2024
2032
USD
4.99 Billion
USD
10.66 Billion
2024
2032
| 2025 –2032 | |
| USD 4.99 Billion | |
| USD 10.66 Billion | |
|
|
|
|
Electro-Medical Devices in Alzheimer’s Treatment Market Size
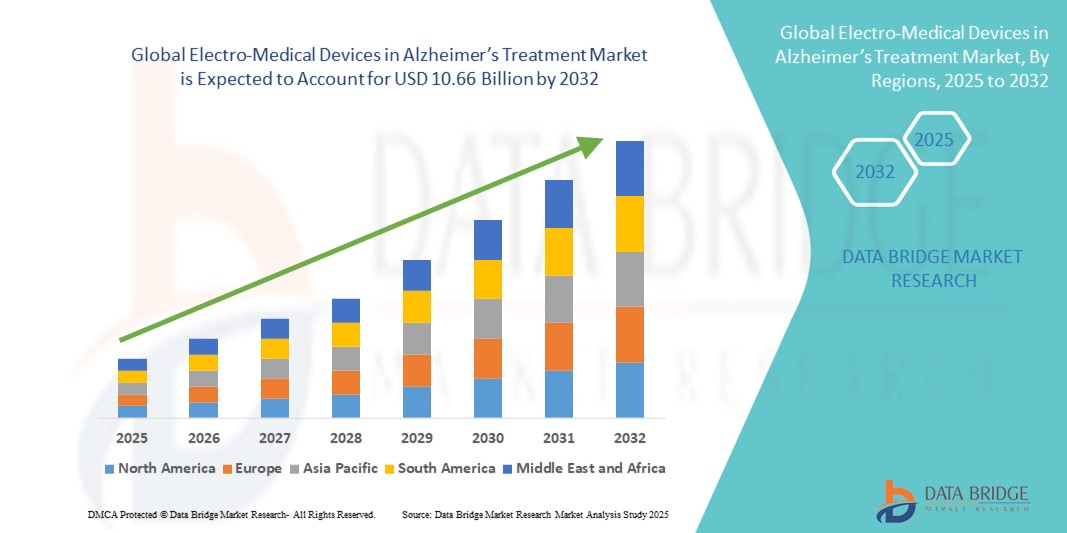
- The global electro-medical devices in Alzheimer’s treatment market size was valued at USD 4.99 billion in 2024 and is expected to reach USD 10.66 billion by 2032, at a CAGR of 9.95% during the forecast period
- The market growth is primarily driven by increasing global prevalence of Alzheimer’s disease and rising demand for non-invasive, technology-driven treatment alternatives that enhance cognitive function and quality of life
- Moreover, continuous innovation in neuromodulation and brain stimulation technologies, combined with growing investments in neurotechnology R&D, is solidifying electro-medical devices as a critical component in Alzheimer’s care. These intersecting trends are accelerating market expansion, significantly propelling the industry's trajectory
Electro-Medical Devices in Alzheimer’s Treatment Market Analysis
- Electro-medical devices, encompassing transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and vagus nerve stimulation (VNS), are becoming essential tools in Alzheimer’s care due to their ability to enhance cognitive function, slow disease progression, and complement traditional drug therapies through non-invasive neural modulation
- The growing demand for these devices is primarily driven by the increasing global prevalence of Alzheimer’s disease, rising geriatric population, and a shift toward non-pharmacological treatment options backed by advancements in neurostimulation technologies
- North America dominated the electro-medical devices in Alzheimer’s treatment market with the largest revenue share of 42.5% in 2024, supported by strong healthcare infrastructure, robust R&D activity, and early adoption of neurotechnology solutions across the U.S. and Canada
- Asia-Pacific is expected to be the fastest growing region in the market during the forecast period due to an aging population, increased healthcare investments, and growing awareness about neurological disorders and device-based interventions
- Cholinesterase Inhibitors segment dominated the electro-medical devices in Alzheimer’s treatment market with a market share of 54.8% in 2024, driven by its proven efficacy in improving cognitive function in early to moderate stages and its widespread use as a first-line therapeutic approach
Report Scope and Electro-Medical Devices in Alzheimer’s Treatment Market Segmentation
|
Attributes |
Electro-Medical Devices in Alzheimer’s Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Electro-Medical Devices in Alzheimer’s Treatment Market Trends
“Technological Advancements in Non-Invasive Neurostimulation Devices”
- A prominent and accelerating trend in the global electro-medical devices market for Alzheimer’s treatment is the advancement and miniaturization of non-invasive neurostimulation technologies, such as transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and wearable neurotech. These innovations are enhancing treatment accessibility, patient comfort, and therapeutic effectiveness
- For instance, companies such as NeuroEM Therapeutics are developing wearable TMS devices for at-home use, aiming to deliver consistent and personalized cognitive therapy through low-intensity electromagnetic fields. Similarly, Fisher Wallace Laboratories offers FDA-cleared neurostimulation headsets that can potentially assist in managing Alzheimer’s symptoms
- Newer devices are incorporating AI algorithms and cloud connectivity to monitor cognitive response patterns and automatically adjust stimulation parameters for optimized results. This personalized approach is becoming more prominent in clinical trials and pilot programs for Alzheimer’s care
- The integration of these devices with mobile apps and cloud platforms allows for real-time data tracking, remote clinician supervision, and user-friendly interfaces—empowering patients and caregivers with greater control over treatment
- These developments are redefining the therapeutic landscape, shifting Alzheimer’s care from facility-based interventions toward patient-centric, at-home management supported by digital therapeutics. Companies such as Cognionics and Neuroelectrics are advancing portable EEG-guided brain stimulation systems that reflect this shift
- The rising demand for intelligent, wearable, and user-friendly electro-medical devices is transforming how Alzheimer’s is treated, with strong momentum from both healthcare providers and tech developers focused on cognitive restoration and quality of life improvement
Electro-Medical Devices in Alzheimer’s Treatment Market Dynamics
Driver
“Rising Alzheimer’s Prevalence and Demand for Non-Pharmacological Alternative”
- The global rise in Alzheimer’s disease cases, fueled by the aging population, is a key driver accelerating demand for innovative treatment options such as electro-medical devices. These solutions offer symptom relief and cognitive stimulation without the side effects associated with long-term drug use
- For instance, in June 2024, Neuroelectrics launched its next-generation Starstim platform for Alzheimer’s clinical trials, aiming to deliver adaptive, brain-state-specific neuromodulation therapies. Such advancements are expected to boost market growth and attract both clinical and commercial interest
- As concerns about limited drug efficacy and side effects persist, the healthcare community is increasingly exploring device-based interventions that offer targeted, repeatable, and personalized treatment often used alongside conventional therapies
- Furthermore, increased public and private investments in neurotechnology and brain health research are enabling more rapid commercialization of such devices, expanding treatment access across clinical and home settings
- The appeal of non-invasive, portable systems that enable at-home use and patient independence is further driving demand among caregivers and healthcare providers, particularly for early and mid-stage Alzheimer’s management
Restraint/Challenge
“Regulatory Barriers and Limited Long-Term Efficacy Data”
- One of the major challenges facing the electro-medical devices market for Alzheimer’s treatment is navigating complex regulatory pathways and demonstrating long-term therapeutic efficacy in a condition known for gradual progression and variability
- For instance, while many neurostimulation devices are FDA-cleared for depression or epilepsy, gaining specific Alzheimer’s indication requires robust clinical trial evidence and long-term outcome data often a resource-intensive process
- Limited availability of large-scale, longitudinal studies supporting sustained cognitive improvement in Alzheimer’s patients using these devices has made some clinicians cautious about wide-scale adoption
- In addition, high device costs and reimbursement uncertainties—especially in emerging markets may deter healthcare providers and patients from considering electro-medical devices as frontline therapy
- Overcoming these challenges through expanded clinical research, international regulatory harmonization, and greater payer coverage will be crucial to ensuring broader acceptance and integration of electro-medical devices into mainstream Alzheimer’s care pathways
Electro-Medical Devices in Alzheimer’s Treatment Market Scope
The market is segmented on the basis of drug class, drug type, and therapeutics.
- By Drug Class
On the basis of drug class, the electro-medical devices in Alzheimer’s treatment market is segmented into cholinergic, memantine, combined drug, AChE inhibitors, and immunoglobulins. The cholinergic segment dominated the market with the largest revenue share of 32.6% in 2024, driven by its role in enhancing neurotransmitter activity to improve cognitive function in mild to moderate stages of Alzheimer’s disease. Cholinergic agents, particularly when paired with neurostimulation techniques, are being increasingly integrated into multimodal treatment protocols that support memory and attention improvement.
The memantine segment is projected to witness the fastest growth rate of 21.3% from 2025 to 2032, due to its effectiveness in moderate to severe stages and its potential for synergistic effects when used in combination with electro-medical therapies. Memantine’s role in modulating glutamatergic signaling is particularly valuable in personalized and combination therapeutic regimens involving device-based stimulation.
- By Drug Type
On the basis of drug type, the electro-medical devices in Alzheimer’s treatment market is segmented into cholinesterase inhibitors and NMDA receptor antagonists. The cholinesterase inhibitors segment held the largest market share of 54.8% in 2024, as these drugs remain the cornerstone of early Alzheimer’s treatment strategies and are frequently administered in conjunction with device-based cognitive stimulation. Their ability to temporarily improve or stabilize symptoms makes them an essential part of dual-therapy interventions, particularly in outpatient settings.
The NMDA receptor antagonists segment is expected to grow at a notable CAGR from 2025 to 2032, driven by their expanding role in managing advanced Alzheimer’s symptoms and increasing clinical validation for use alongside transcranial stimulation technologies.
- By Therapeutics
On the basis of therapeutics, the electro-medical devices in Alzheimer’s treatment market is segmented into cholinesterase inhibitors, NMDA receptor antagonists, and other therapeutics. The cholinesterase inhibitors segment dominated the therapeutics category with a market share of 51.7% in 2024, owing to their wide prescription base, proven cognitive benefits, and compatibility with electro-medical devices in structured treatment plans. These therapeutics are preferred in early-stage Alzheimer’s cases where neurostimulation complements pharmacological support.
The NMDA receptor antagonists segment is expected to exhibit the fastest growth over the forecast period, supported by growing research interest in combining pharmacological NMDA modulation with neuro-electrical therapies to achieve enhanced synaptic plasticity and memory retention outcomes.
Electro-Medical Devices in Alzheimer’s Treatment Market Regional Analysis
- North America dominated the electro-medical devices in Alzheimer’s treatment market with the largest revenue share of 42.5% in 2024, supported by strong healthcare infrastructure, robust R&D activity, and early adoption of neurotechnology solutions across the U.S. and Canada
- Patients and caregivers in the region increasingly value the availability of non-invasive, technology-driven treatment alternatives that complement or substitute pharmacological approaches, especially in early to moderate stages of the disease
- This strong market presence is further supported by substantial R&D investment, favorable reimbursement policies, and a well-established regulatory framework, positioning electro-medical devices as a key component in integrated Alzheimer’s care across hospitals, clinics, and home-care environments
U.S. Electro-Medical Devices in Alzheimer’s Treatment Market Insight
The U.S. electro-medical devices market for Alzheimer’s treatment captured the largest revenue share of 83% in North America in 2024, driven by the country’s advanced healthcare infrastructure and robust investment in neurotechnology research. Widespread clinical adoption of non-invasive brain stimulation devices and favorable FDA support for neuromodulation trials are accelerating market growth. In addition, the rise in Alzheimer’s cases and increasing demand for home-based therapeutic solutions are encouraging the integration of wearable and portable electro-medical devices across care settings.
Europe Electro-Medical Devices in Alzheimer’s Treatment Market Insight
The Europe market is projected to grow at a significant CAGR throughout the forecast period, driven by strong governmental and institutional support for dementia care innovation and growing clinical trials involving electro-medical interventions. Increasing awareness of non-drug therapies and favorable reimbursement policies are supporting adoption across hospital and outpatient settings. Rising Alzheimer’s prevalence, particularly in Western and Northern Europe, is also pushing the demand for cognitive stimulation and neuromodulation technologies.
U.K. Electro-Medical Devices in Alzheimer’s Treatment Market Insight
The U.K. market is anticipated to grow at a notable CAGR during the forecast period, fueled by national initiatives for dementia care and digital health integration. With an aging population and growing concern for cognitive health, the U.K. is expanding its adoption of device-based Alzheimer’s therapies, especially in NHS-supported research and community care programs. Integration with AI-enabled monitoring systems is gaining traction as part of the country’s long-term care strategy.
Germany Electro-Medical Devices in Alzheimer’s Treatment Market Insight
Germany is expected to witness considerable market growth, supported by its strong medtech innovation ecosystem and increasing investments in neurological research. The country’s emphasis on data security and medical device compliance aligns well with the development of safe and effective electro-medical devices. Growing use of brain stimulation in clinical trials and academic hospitals is paving the way for wider clinical application in Alzheimer’s therapy.
Asia-Pacific Electro-Medical Devices in Alzheimer’s Treatment Market Insight
The Asia-Pacific market is expected to register the fastest CAGR of 24% from 2025 to 2032, driven by a surging elderly population, growing awareness of neurodegenerative disorders, and expanding healthcare access in countries such as China, Japan, and India. Government-backed initiatives to address dementia and increasing participation in international clinical trials are boosting regional adoption. The growing number of local device manufacturers is also contributing to improved affordability and accessibility.
Japan Electro-Medical Devices in Alzheimer’s Treatment Market Insight
Japan’s market is gaining momentum due to the country’s strong focus on elderly care and high technological sophistication. The integration of electro-medical devices with robotics, AI, and smart home technologies is enhancing Alzheimer’s patient management. Government support for dementia research and assistive technologies is further fueling growth, particularly in home-care and assisted living facilities.
India Electro-Medical Devices in Alzheimer’s Treatment Market Insight
India accounted for the largest revenue share in Asia-Pacific in 2024, supported by its rapidly aging population, expanding private healthcare sector, and increasing demand for cost-effective Alzheimer’s treatment solutions. With growing domestic innovation and rising awareness of cognitive health, India is emerging as a key market for wearable and affordable neurostimulation devices. Government-led smart health and geriatric care initiatives are expected to sustain strong market momentum.
Electro-Medical Devices in Alzheimer’s Treatment Market Share
The electro-medical devices in Alzheimer’s treatment industry is primarily led by well-established companies, including:
- Cognito Therapeutics, Inc. (U.S.)
- Neuroelectrics (Spain)
- Fisher Wallace Laboratories, Inc. (U.S.)
- NeuroEM Therapeutics, Inc. (U.S.)
- MagVenture A/S (Denmark)
- Neuronetics, Inc. (U.S.)
- Soterix Medical Inc. (U.S.)
- BrainsWay Ltd. (Israel)
- Flow Neuroscience AB (Sweden)
- Ybrain Inc. (South Korea)
- eNeura Inc. (U.S.)
- TMS Neuro Solutions (U.S.)
- Helius Medical Technologies, Inc. (U.S.)
- NeuroSigma, Inc. (U.S.)
- NeuroPace, Inc. (U.S.)
- Magstim Company Ltd. (U.K.)
- Bittium Corporation (Finland)
- Nexalin Technology, Inc. (U.S.)
- EB Neuro S.p.A. (Italy)
- Neurosoft (Russia)
What are the Recent Developments in Global Electro-Medical Devices in Alzheimer’s Treatment Market?
- In June 2024, Neuroelectrics, a pioneering neurotechnology company, launched the next-generation Starstim-Home platform for Alzheimer’s clinical use, enabling non-invasive brain stimulation in home settings. This platform offers remote monitoring, real-time data transmission, and tailored stimulation protocols aimed at improving cognitive function. The innovation highlights the growing trend toward personalized and decentralized neuromodulation therapies, enhancing accessibility and convenience for patients and caregivers while supporting clinical research
- In April 2024, Cognito Therapeutics announced the expansion of its Phase III clinical trial for its wearable neurostimulation device, which uses gamma frequency light and sound to target Alzheimer’s pathology. Backed by FDA Breakthrough Device designation, the device has shown promise in reducing brain atrophy and improving memory function. This development signifies increasing investment in wearable neuro-devices as viable treatment modalities for neurodegenerative diseases
- In March 2024, Fisher Wallace Laboratories reported progress in its pilot programs using its FDA-cleared Stimulator device to support cognitive health in early-stage Alzheimer’s patients. The device delivers transcranial electrical stimulation (tES) and is being assessed for its ability to reduce symptoms such as agitation and sleep disturbances, common in Alzheimer’s care. This effort highlights growing clinical interest in repurposing approved neuromodulation technologies for dementia-related applications
- In February 2024, NeuroEM Therapeutics received additional funding from the Alzheimer’s Drug Discovery Foundation (ADDF) to accelerate development of its MemorEM wearable device. Designed to deliver electromagnetic treatments targeting amyloid plaques, the headset has completed successful early-stage trials demonstrating cognitive improvement. This advancement reflects the expanding role of electro-medical wearables in targeting disease mechanisms beyond symptomatic relief
- In January 2024, Magstim, a leader in transcranial magnetic stimulation (TMS), announced a collaboration with European research institutes to explore repetitive TMS as a therapeutic tool for Alzheimer’s patients. The initiative aims to refine stimulation protocols and assess their long-term effects on memory and neuroplasticity. This collaboration underscores the growing integration of academic research and commercial innovation in advancing electro-medical Alzheimer’s treatments globally
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.