Global Elisa Tests Market
Market Size in USD Billion
CAGR :
% 
 USD
2.29 Billion
USD
4.00 Billion
2025
2033
USD
2.29 Billion
USD
4.00 Billion
2025
2033
| 2026 –2033 | |
| USD 2.29 Billion | |
| USD 4.00 Billion | |
|
|
|
|
ELISA Tests Market Size
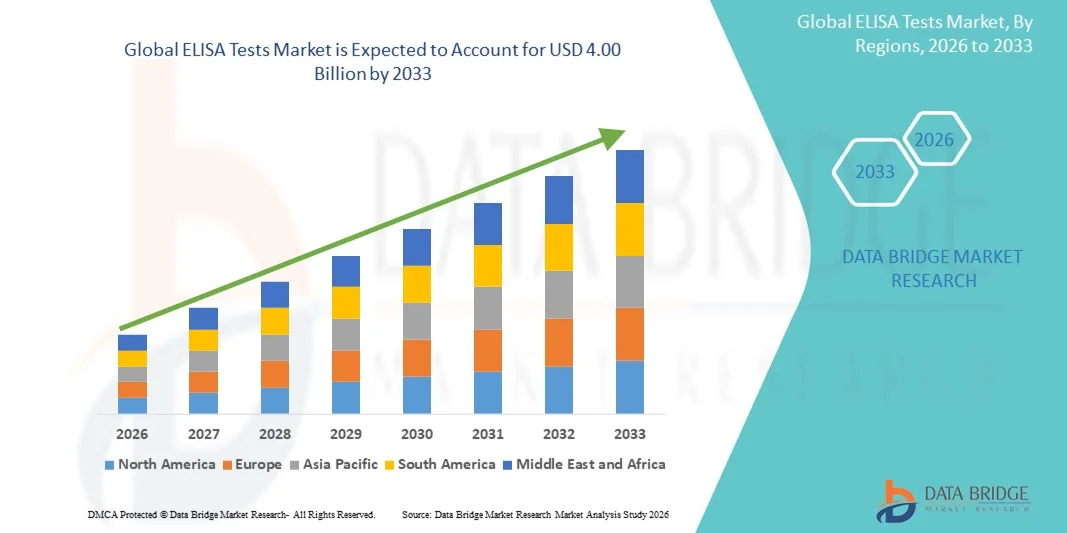
- The global ELISA tests market size was valued at USD 2.29 billion in 2025 and is expected to reach USD 4.00 billion by 2033, at a CAGR of 7.23% during the forecast period
- The market growth is primarily driven by increasing prevalence of infectious and chronic diseases, growing demand for accurate and high-throughput diagnostic tools, and advancements in immunoassay technologies
- Moreover, rising awareness about early disease detection, expanding research and clinical diagnostics applications, and the integration of automated and miniaturized ELISA platforms are further propelling the adoption of ELISA tests, thereby significantly enhancing the market’s growth potential
ELISA Tests Market Analysis
- ELISA tests, offering highly sensitive and specific immunoassay-based detection of antigens and antibodies, are increasingly essential tools in clinical diagnostics, research laboratories, and pharmaceutical applications due to their accuracy, scalability, and adaptability across various disease targets
- The escalating demand for ELISA tests is primarily driven by the rising prevalence of infectious and chronic diseases, growing focus on early disease detection, and the need for reliable high-throughput diagnostic solutions
- North America dominated the ELISA tests market with the largest revenue share of 38.7% in 2025, attributed to well-established healthcare infrastructure, high adoption of advanced diagnostic technologies, and strong presence of leading market players, with the U.S. witnessing substantial growth in ELISA applications across clinical diagnostics, vaccine development, and research, fueled by technological advancements and automation in testing platforms
- Asia-Pacific is expected to be the fastest-growing region in the ELISA tests market during the forecast period due to increasing healthcare investments, expanding research activities, and rising awareness about early disease detection
- Sandwich ELISA segment dominated the ELISA tests market with a market share of 41.5% in 2025, driven by its high sensitivity, specificity, and wide applicability in detecting low-concentration biomarkers in clinical and research settings
Report Scope and ELISA Tests Market Segmentation
|
Attributes |
ELISA Tests Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
ELISA Tests Market Trends
Automation and High-Throughput Integration
- A major and growing trend in the global ELISA tests market is the adoption of automated and high-throughput ELISA platforms, which streamline sample processing, reduce manual errors, and increase testing efficiency
- For instance, the Dynex DS2 ELISA system automates multiple assay steps, allowing laboratories to handle hundreds of samples with minimal human intervention, improving throughput and reproducibility
- Integration with laboratory information management systems (LIMS) enables seamless data collection, analysis, and reporting, supporting faster decision-making in clinical diagnostics and research
- High-throughput ELISA systems also facilitate large-scale screening for infectious diseases, vaccine development, and biomarker research, meeting the growing demand for rapid and reliable diagnostic results
- This trend towards automation and high-throughput capabilities is fundamentally transforming laboratory workflows and raising expectations for efficiency, accuracy, and scalability in ELISA testing
- The demand for automated ELISA platforms is expanding across clinical laboratories, research institutions, and pharmaceutical companies, driven by the need for faster, more standardized, and reliable testing processes
- Integration of ELISA platforms with cloud-based data analytics is gaining momentum, allowing real-time monitoring of assay performance and large-scale epidemiological studies
- Emerging miniaturized and portable ELISA devices are enabling on-site testing in field conditions and decentralized labs, expanding access to diagnostics in remote or resource-limited regions
ELISA Tests Market Dynamics
Driver
Rising Disease Prevalence and Demand for Early Detection
- The increasing incidence of infectious and chronic diseases, along with heightened focus on early diagnosis, is a significant driver for the growing adoption of ELISA tests
- For instance, the CDC reported a surge in viral and bacterial infections in 2025, highlighting the need for sensitive diagnostic tools such as ELISA for timely detection and treatment
- ELISA tests offer high specificity and sensitivity, enabling accurate detection of disease biomarkers even at low concentrations, making them vital for early-stage diagnostics
- Furthermore, the growing investment in preventive healthcare and screening programs is promoting wider adoption of ELISA assays in both hospital and laboratory settings
- Continuous advancements in ELISA technology, such as multiplex assays and miniaturized platforms, further enhance the speed, accuracy, and usability of these tests, driving market growth
- Rising research activities in immunology, oncology, and infectious diseases are increasing demand for ELISA assays for biomarker discovery and drug development
- Integration with other diagnostic technologies, such as PCR and lateral flow assays, is enhancing the versatility and applicability of ELISA tests across multi-step diagnostic workflows
Restraint/Challenge
High Cost and Standardization Barriers
- The relatively high cost of advanced ELISA systems and reagents, along with the need for skilled personnel, can limit adoption in resource-constrained laboratories and developing regions
- For instance, some automated multiplex ELISA platforms require substantial initial investment and trained operators, posing a barrier for smaller clinical or research labs
- In addition, variability in assay protocols and lack of standardized procedures across laboratories can affect reproducibility and comparability of results, limiting broader market penetration
- While ELISA remains a gold standard for immunoassays, newer low-cost point-of-care alternatives and rapid diagnostic tests are emerging, challenging traditional ELISA adoption in certain settings
- Addressing these challenges through cost-effective solutions, standardized assay protocols, and training initiatives will be critical for sustaining market growth
- Manufacturers such as Thermo Fisher Scientific and Bio-Rad are focusing on developing user-friendly, affordable, and standardized ELISA platforms to overcome these barriers and expand market reach
- Regulatory hurdles and lengthy approval processes for diagnostic assays can delay market entry of new ELISA kits, impacting commercialization timelines
- Dependence on high-quality reagents and consistent supply chains poses operational challenges, particularly in regions with limited infrastructure or geopolitical constraints
ELISA Tests Market Scope
The market is segmented on the basis of method, application, technology, and end user
- By Method
On the basis of method, the ELISA tests market is segmented into Direct ELISA, Indirect ELISA, Sandwich ELISA, and Competitive ELISA. The Sandwich ELISA segment dominated the market with the largest market revenue share of 41.5% in 2025, driven by its high sensitivity and specificity in detecting low-concentration antigens. Sandwich ELISA is widely used in clinical diagnostics, vaccine development, and research laboratories due to its ability to accurately quantify target proteins and antibodies. Laboratories prefer this method for its robustness and reproducibility, especially when analyzing complex biological samples. Its compatibility with automated and high-throughput platforms also supports large-scale testing, which further reinforces its market dominance. The method is particularly popular in infectious disease screening, oncology biomarker detection, and immunology research, providing reliable results across a broad range of applications.
The Indirect ELISA segment is anticipated to witness the fastest growth rate of 22.1% from 2026 to 2033, fueled by its versatility and cost-effectiveness. Indirect ELISA is preferred for antibody detection in immunology studies, epidemiological surveys, and serological testing. Its lower reagent consumption compared to other methods and the ability to amplify signals through secondary antibodies make it an attractive choice for research and diagnostic applications. In addition, rising demand for large-scale screening of immune responses in vaccine trials and disease surveillance is contributing to the rapid adoption of indirect ELISA globally.
- By Application
On the basis of application, the ELISA tests market is segmented into vaccine development, immunology, diagnostics, toxicology, drug monitoring, pharmaceutical industry, transplantation, infectious diseases, cancer, protein quantitation, and others. The Diagnostics segment dominated the market with the largest revenue share in 2025, driven by the rising prevalence of infectious and chronic diseases and the growing emphasis on early and accurate disease detection. ELISA assays are extensively used in clinical laboratories for diagnosing conditions such as HIV, hepatitis, and autoimmune disorders. Their high sensitivity, reproducibility, and compatibility with automation enable laboratories to process large numbers of patient samples efficiently. Diagnostic applications also benefit from technological advancements such as chemiluminescent and fluorescent ELISA, which provide faster and more precise results.
The Vaccine Development segment is expected to witness the fastest growth rate of 23.4% during 2026–2033, driven by the increasing number of clinical trials, emerging infectious diseases, and the demand for rapid immunogenicity testing. ELISA is critical in evaluating antibody responses, quantifying antigen levels, and monitoring vaccine efficacy. Pharmaceutical companies and research institutes are investing heavily in ELISA-based high-throughput platforms to accelerate vaccine R&D. The adoption of automated ELISA systems in vaccine production pipelines further enhances efficiency and scalability, making this segment one of the fastest-growing applications in the market.
- By Technology
On the basis of technology, the ELISA tests market is segmented into chemiluminescent, colorimetric, and fluorescent. The Colorimetric segment dominated the market in 2025 due to its simplicity, affordability, and widespread use in both research and diagnostic laboratories. Colorimetric ELISA provides clear visual readouts and is compatible with conventional microplate readers, making it accessible to laboratories of all sizes. Its reliability and ease of implementation make it a preferred choice for routine clinical diagnostics and protein quantitation assays. Furthermore, its ability to generate reproducible quantitative results at a low operational cost supports its continued dominance.
The Chemiluminescent segment is anticipated to witness the fastest growth rate of 24.0% from 2026 to 2033, driven by its higher sensitivity, wider dynamic range, and suitability for automation and high-throughput screening. Chemiluminescent ELISA is increasingly adopted in advanced diagnostics, oncology research, and infectious disease testing due to its ability to detect low-abundance biomarkers with high precision. Integration with automated platforms and LIMS systems further accelerates its uptake in large clinical laboratories and research institutions, contributing to rapid market growth.
- By End User
On the basis of end user, the ELISA tests market is segmented into hospitals and diagnostic centers, and research laboratories. The Hospitals and Diagnostic Centers segment dominated the market with the largest revenue share in 2025, owing to the increasing number of diagnostic tests conducted for disease detection, monitoring, and preventive healthcare. Hospitals benefit from ELISA’s reliability, accuracy, and compatibility with automated systems, enabling efficient patient sample processing. The segment also leverages ELISA assays for infectious disease screening, oncology biomarker detection, and therapeutic monitoring, ensuring broad applicability across clinical departments.
The Research Laboratories segment is expected to witness the fastest growth rate of 21.9% during 2026–2033, driven by expanding research activities in immunology, oncology, vaccine development, and drug discovery. Research institutions increasingly adopt advanced and miniaturized ELISA platforms for high-throughput screening, biomarker quantitation, and experimental studies. The rising demand for rapid, reproducible, and cost-effective assays to support scientific research is fueling the segment’s growth globally.
ELISA Tests Market Regional Analysis
- North America dominated the ELISA tests market with the largest revenue share of 38.7% in 2025, attributed to well-established healthcare infrastructure, high adoption of advanced diagnostic technologies, and strong presence of leading market players
- Laboratories and hospitals in the region increasingly rely on ELISA assays for early disease detection, vaccine development, and biomarker research, owing to their high sensitivity, specificity, and reproducibility
- This widespread adoption is further supported by significant investments in research and development, availability of skilled healthcare professionals, and strong presence of key market players, establishing ELISA as a preferred diagnostic and research tool across clinical and laboratory settings
U.S. ELISA Tests Market Insight
The U.S. ELISA tests market captured the largest revenue share of 82% in 2025 within North America, fueled by the high prevalence of infectious and chronic diseases and the widespread adoption of advanced diagnostic technologies. Clinical laboratories and hospitals increasingly rely on ELISA assays for early detection, vaccine development, and biomarker research. The strong focus on preventive healthcare, combined with significant R&D investments and the presence of major market players, further propels market growth. Moreover, the integration of automated and high-throughput ELISA systems enhances testing efficiency, accuracy, and reproducibility, driving widespread adoption across healthcare and research institutions.
Europe ELISA Tests Market Insight
The Europe ELISA tests market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by stringent regulatory standards for diagnostics and growing awareness of early disease detection. The adoption of ELISA assays is increasing across hospitals, diagnostic centers, and research institutions, supported by advanced healthcare infrastructure. Rising investments in biotechnology and pharmaceutical research are fostering market growth. In addition, the demand for multiplex and high-sensitivity ELISA assays for oncology, infectious disease, and immunology applications is significantly contributing to market expansion.
U.K. ELISA Tests Market Insight
The U.K. ELISA tests market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising healthcare expenditure, growing prevalence of chronic and infectious diseases, and the demand for early, accurate diagnostics. Hospitals and research laboratories are increasingly adopting ELISA for clinical testing, vaccine trials, and biomarker studies. Furthermore, the U.K.’s robust pharmaceutical research sector, coupled with advanced laboratory infrastructure and skilled workforce, continues to stimulate market growth. Adoption of automated ELISA platforms is also accelerating, enhancing testing efficiency and reproducibility across healthcare and research settings.
Germany ELISA Tests Market Insight
The Germany ELISA tests market is expected to expand at a considerable CAGR during the forecast period, fueled by high awareness of healthcare diagnostics and advanced technological infrastructure. German hospitals and laboratories prioritize ELISA assays for clinical diagnostics, immunology research, and protein quantitation due to their sensitivity and reliability. Strong government support for healthcare innovation and investment in biotechnology research further drives adoption. In addition, integration of automated and high-throughput ELISA platforms is becoming increasingly prevalent, supporting large-scale testing and improving operational efficiency.
Asia-Pacific ELISA Tests Market Insight
The Asia-Pacific ELISA tests market is poised to grow at the fastest CAGR of 23.8% during the forecast period of 2026 to 2033, driven by increasing healthcare expenditure, rising prevalence of infectious diseases, and expanding research activities in countries such as China, Japan, and India. Rapid urbanization, growing healthcare infrastructure, and increasing awareness of early disease detection are boosting ELISA adoption. Furthermore, the region is emerging as a hub for manufacturing ELISA kits and reagents, improving affordability and accessibility across clinical and research laboratories.
Japan ELISA Tests Market Insight
The Japan ELISA tests market is gaining momentum due to the country’s technologically advanced healthcare infrastructure and high adoption of diagnostic innovations. Clinical laboratories and hospitals increasingly use ELISA assays for infectious disease testing, oncology biomarker detection, and vaccine development. Integration with automated platforms enhances throughput and accuracy, supporting widespread adoption. Furthermore, Japan’s aging population is driving demand for reliable, rapid diagnostic solutions, particularly in hospitals and research institutions focusing on chronic disease management and immunology studies.
India ELISA Tests Market Insight
The India ELISA tests market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to rising healthcare awareness, expanding diagnostic facilities, and increasing prevalence of infectious diseases. Hospitals, diagnostic centers, and research laboratories are increasingly adopting ELISA assays for early detection, vaccine trials, and biomarker research. Government initiatives promoting affordable healthcare diagnostics, coupled with domestic manufacturing of ELISA kits and reagents, are improving accessibility. The growing focus on preventive healthcare and expansion of private diagnostic services are key factors propelling market growth in India.
ELISA Tests Market Share
The ELISA Tests industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Danaher (U.S.)
- PerkinElmer (U.S.)
- Siemens Healthineers AG (Germany)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Abbott (U.S.)
- Merck KGaA (Germany)
- BD (U.S.)
- Bio-Techne Corporation (U.S.)
- Enzo Biochem, Inc. (U.S.)
- RayBiotech, Inc. (U.S.)
- OriGene Technologies, Inc. (U.S.)
- Creative Diagnostics (U.S.)
- MyBioSource, Inc. (U.S.)
- Abcam plc (U.K.)
- Eurofins Scientific (Luxembourg)
- Elabscience Biotechnology Inc. (U.S.)
- GenScript (China)
- BioVision, Inc. (U.S.)
What are the Recent Developments in Global ELISA Tests Market?
- In October 2025, Creative Diagnostics launched a validated suite of sandwich ELISA kits tailored for mRNA vaccine/therapeutic production quality‑control including inorganic pyrophosphatase (PPase) detection, dsRNA quantitation, and Protein A leachate monitoring
- In April 2024, Creative Diagnostics also announced the launch of an expanded portfolio of ELISA‑based assays for vaccine development and bioprocess applications, including viral vector titer determination (AAV, lentivirus p24), antigen/antibody detection and immunogenicity measurement
- In April 2024, Bio Techne Corporation, through its brand R&D Systems, was awarded the “ELISA Kit Supplier of the Year” by CiteAb, recognising its ELISA kits’ wide citations in research literature and quality validation. The award underscores the brand’s leadership in providing robust, reproducible ELISA assays with over 1 000 targets across 16 species
- In February 2023, Charles River Laboratories launched its first IgY‑based ELISA kit for detection and quantitation of residual host cell proteins (HCP) in CHO‑based biotherapeutics. This kit uses chicken immunoglobulin Y (IgY) antibodies to improve impurity coverage (up to ~90 %) and sensitivity down to 0.1 ng/mL, addressing a major quality control gap in biologics manufacturing
- In May 2021, the Indian start‑up PathShodh Healthcare announced a novel point‑of‑care electrochemical ELISA test for COVID‑19 IgM/IgG antibodies that enables semi‑quantitative estimation of antibody concentration in clinical samples
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.