Global Elispot Market
Market Size in USD Billion
CAGR :
% 
 USD
1.06 Billion
USD
2.47 Billion
2025
2033
USD
1.06 Billion
USD
2.47 Billion
2025
2033
| 2026 –2033 | |
| USD 1.06 Billion | |
| USD 2.47 Billion | |
|
|
|
|
Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Size
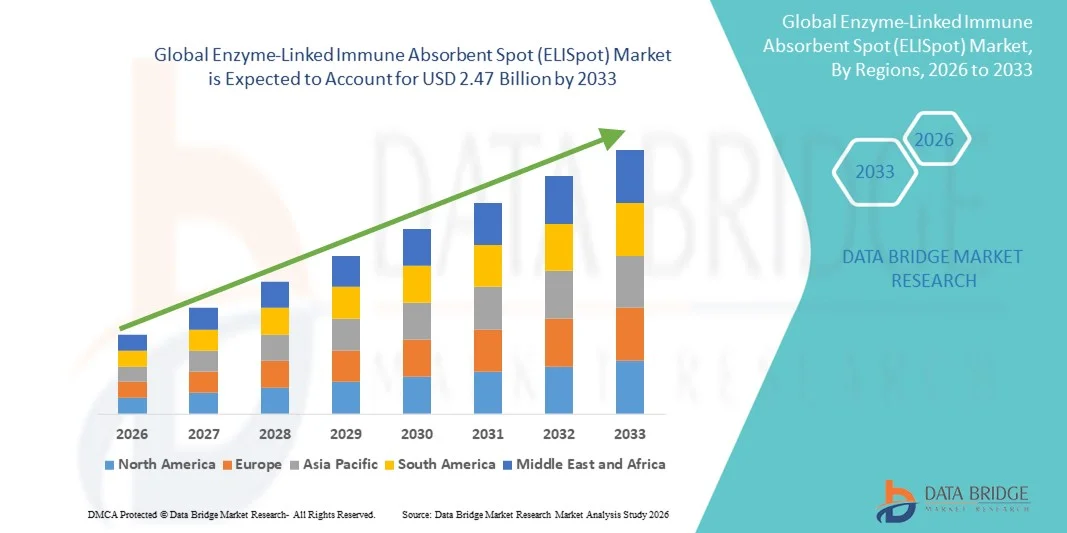
- The global Enzyme-Linked Immune Absorbent Spot (ELISpot) market size was valued at USD 1.06 billion in 2025 and is expected to reach USD 2.47 billion by 2033, at a CAGR of 11.20% during the forecast period
- The market growth is largely fueled by the growing adoption of advanced immunological assays for high-sensitivity T-cell and B-cell analysis, alongside continuous technological progress in immunodiagnostics, thereby driving increased utilization across infectious disease monitoring, vaccine development, autoimmune research, and oncology
- Furthermore, rising global demand for precise, reliable, and high-throughput immune-monitoring solutions is positioning ELISpot as a preferred assay platform within clinical research and translational medicine. These converging factors are accelerating the adoption of ELISpot systems and kits, thereby significantly boosting the industry's growth
Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Analysis
- ELISpot assays, enabling precise detection of cytokine-secreting immune cells, are increasingly vital components of modern immunology workflows across vaccine development, infectious disease research, autoimmune studies, and cancer immunotherapy due to their exceptional sensitivity, functional specificity, and ability to characterize cell-mediated immune responses at a single-cell level
- The escalating demand for ELISpot technologies is primarily fueled by the growing global emphasis on immune-monitoring in clinical trials, rising prevalence of infectious and chronic diseases, and expanding adoption of high-throughput immunoassays by biopharmaceutical companies, research institutes, and CROs
- North America dominated the ELISpot market with the largest revenue share of 38.9% in 2025, supported by advanced biotechnology infrastructure, high R&D investments, and strong presence of leading assay developers, with the U.S. experiencing substantial growth in ELISpot utilization across vaccine pipelines, immunotherapy trials, and academic immunology programs
- Asia-Pacific is expected to be the fastest growing region in the ELISpot market during the forecast period due to rapid expansion of clinical research activities, increasing government funding for infectious disease control, and the emergence of strong biotech clusters in China, India, South Korea, and Singapore
- T-Cell-Based Kits segment dominated the ELISpot market with a market share of 46.5% in 2025, driven by their widespread use in evaluating antigen-specific immune responses, strong acceptance as a gold-standard method for cellular immunity assessment, and critical role in vaccine development and immuno-oncology studies
Report Scope and Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Segmentation
|
Attributes |
Enzyme-Linked Immune Absorbent Spot (ELISpot) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Trends
Rising Adoption of Automated and AI-Enhanced Immune Monitoring Platforms
- A significant and accelerating trend in the global ELISpot market is the deepening integration of automation technologies and AI-driven analytical tools to enhance precision, reproducibility, and throughput in immune-cell analysis across vaccine development, infectious disease research, and immuno-oncology
- For instance, automated ELISpot analyzers such as the ImmunoSpot® platform integrate AI-assisted spot-counting algorithms, enabling researchers to rapidly quantify cytokine-secreting cells with improved accuracy and reduced operator variability, thereby strengthening assay standardization in clinical research
- AI integration within advanced ELISpot systems enables pattern recognition, intelligent assay optimization, and enhanced quality control by detecting inconsistencies in spot morphology or plate performance, while predictive algorithms assist researchers in identifying atypical immune responses during early-stage trials
- The seamless integration of ELISpot platforms with digital laboratory ecosystems facilitates unified data management, remote assay monitoring, and streamlined workflow automation, allowing labs to coordinate ELISpot results alongside flow cytometry, ELISA, and sequencing data within a centralized environment
- This trend toward intelligent, automated, and interoperable immune-monitoring systems is fundamentally reshaping expectations for assay precision and efficiency in immunology research, encouraging companies such as CTL and Oxford Immunotec to develop next-generation AI-enabled ELISpot platforms offering enhanced analysis capabilities and remote system connectivity
- The demand for ELISpot systems that offer automation, AI-enhanced analytics, and seamless integration with broader laboratory information systems is growing rapidly across biopharmaceutical companies, CROs, and academic research centers as they increasingly prioritize high-throughput, standardized immune-response analysis
Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Dynamics
Driver
Growing Need Due to Rising Vaccine Development and Immunotherapy Research
- The increasing prevalence of infectious and chronic diseases, combined with rapid expansion in vaccine development pipelines and immunotherapy programs, is a significant driver for the heightened adoption of ELISpot assays as essential tools for cellular immune-response evaluation
- For instance, in April 2025, Oxford Immunotec announced strategic enhancements to its T-SPOT platform to support large-scale clinical trial immune-monitoring needs, enabling more efficient processing of high-volume ELISpot assays; such advancements by key companies are expected to drive market growth in the forecast period
- As research organizations and biopharmaceutical companies intensify their focus on characterizing antigen-specific T-cell responses, ELISpot provides advantages such as high sensitivity, single-cell resolution, and the ability to track immune functionality, making it a compelling upgrade over traditional serological or bulk cytokine assays
- Furthermore, the growing popularity of cell-based functional assays and the rising demand for comprehensive immune-monitoring workflows are making ELISpot an integral component of clinical and translational research, supported by increasing integration with automated analyzers and data platforms
- The ability to perform high-precision immune monitoring across vaccine trials, oncology studies, transplant immunology, and infectious disease programs is a key factor propelling the adoption of ELISpot assays across CROs, research institutions, and biopharma companies. The trend toward decentralized and standardized immune-response testing further contributes to market growth
Restraint/Challenge
Operational Complexity and Regulatory Compliance Hurdle
- Concerns surrounding assay complexity, variability in manual interpretation, and challenges in regulatory compliance pose significant hurdles to wider ELISpot implementation, particularly in high-throughput or clinical settings where standardization and reproducibility are critical
- For instance, reports highlighting inconsistencies in ELISpot spot-counting due to manual plate handling or subjective interpretation have made some clinical laboratories hesitant to adopt ELISpot workflows without advanced automated analysis tools
- Addressing these concerns through automated plate readers, AI-driven spot-counting algorithms, and robust quality-control systems is crucial for building confidence among clinical researchers. Companies such as CTL and Mabtech emphasize their advanced standardization and automation technologies to reassure potential users about assay reliability
- In addition, the relatively high cost associated with sophisticated ELISpot analyzers, high-quality kits, and laboratory automation systems can be a barrier to adoption for smaller research organizations or labs in developing regions. While basic ELISpot kits have become more accessible, platforms with advanced automation or multiplexing capabilities often carry premium pricing
- While technology advancements are gradually reducing manual workload, the perceived complexity and operational expertise required to run ELISpot assays can still hinder widespread adoption, especially among laboratories without established immunology infrastructure
- Overcoming these challenges through enhanced automation, user-friendly assay formats, operator training, and more cost-effective ELISpot solutions will be essential for sustained market growth
Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Scope
The market is segmented on the basis of utility, analyte, application, and end-users.
- By Utility
On the basis of utility, the ELISpot market is segmented into diagnostic kits and research kits. The diagnostic kits segment held the largest market share in 2025 due to their widespread use in infectious disease diagnostics, vaccine evaluation, and immunodeficiency assessment. These kits are frequently utilized by hospitals, public health labs, and clinical diagnostic centers for standardized and validated immunological testing. Growth is supported by rising global emphasis on early disease detection and immune monitoring especially for tuberculosis, viral infections, and post-transplant immune assessment. In addition, diagnostic ELISpot kits benefit from stringent regulatory approvals, which enhance their reliability and adoption across high-compliance clinical environments. As personalized medicine expands, diagnostic ELISpot kits continue to gain relevance in monitoring patient-specific immune responses.
The research kits segment is expected to witness the highest CAGR from 2026 to 2033, driven by expanding immunology research, vaccine discovery programs, and oncology pipeline growth. Research institutions increasingly rely on ELISpot kits to quantify cytokine secretion at the single-cell level an essential step in evaluating T-cell and B-cell responses. The surge in preclinical and translational research projects, especially in cancer immunotherapy and autoimmune disease mechanisms, is accelerating demand. Furthermore, these kits support customized assay development, offering flexibility that researchers value for exploratory studies. Government and biopharma R&D investments are rising substantially, strengthening the long-term outlook for research kit expansion.
- By Analyte
On the basis of analyte, the ELISpot market is segmented into T-cell-based kits, B-cell-based kits, and others. T-cell-based ELISpot kits accounted for the largest market share of 46.5% in 2025 owing to their critical role in vaccine development, infectious disease monitoring, and immuno-oncology research. These kits enable detection of cytokines such as IFN-γ, IL-2, and TNF-α, which are key indicators of cellular immunity. Their clinical relevance increased substantially due to global interest in T-cell immunity following COVID-19, boosting adoption in both clinical and research sectors. T-cell ELISpot assays are also standard tools in transplant immunology, helping monitor graft response and rejection risks. Their high sensitivity and ability to quantify rare antigen-responsive T cells further reinforce their demand across multiple therapeutic domains.
B-cell-based ELISpot kits are expected to register the fastest growth from 2026 to 2033, fueled by increasing focus on antibody-secreting cell research and vaccine-induced humoral immunity. These assays are essential in evaluating memory B-cell responses, which are becoming increasingly important for long-term vaccine efficacy assessments. The rise of complex biologics, monoclonal antibodies, and autoimmune disease research is creating new opportunities for B-cell-specific immune profiling. In addition, advances in adaptive immunity studies are pushing laboratories to adopt B-cell ELISpot as a complementary tool alongside T-cell assays. As next-generation vaccines and immunotherapies continue expanding, B-cell assay adoption is accelerating rapidly.
- By Application
On the basis of application, the ELISpot market is segmented into diagnostic applications and research applications. Research applications dominated the market in 2025 due to high utilization of ELISpot assays in immunology, oncology, virology, and vaccine research. The technique provides unmatched sensitivity for studying cell-mediated immune responses at the single-cell level, making it indispensable in both academic and biopharma laboratories. Increased pipeline activity in cancer immunotherapies, including checkpoint inhibitors and CAR-T therapies, is driving assay adoption. The method also supports regulatory submissions as ELISpot data is often required by agencies for vaccine and immunotherapy trials. The surge in translational research programs worldwide ensures sustained demand for ELISpot in research-driven environments.
Diagnostic applications are projected to grow at the highest rate from 2026 to 2033, boosted by increasing adoption of ELISpot for tuberculosis screening, viral infection monitoring, and transplant diagnostics. Healthcare systems are placing greater emphasis on immune monitoring, especially in chronic disease patients, which strengthens the role of ELISpot-based diagnostics. The method’s ability to identify antigen-specific immune responses makes it superior to conventional serological tests in many clinical scenarios. Countries with high TB incidence continue integrating ELISpot into national screening programs, further accelerating uptake. In addition, post-infection and post-vaccination immune monitoring demand is rising, supporting rapid diagnostic expansion.
- By End-Users
On the basis of end-users, the ELISpot market is segmented into hospitals & clinical laboratories, research institutes, and biopharmaceutical companies. Research institutes accounted for the largest market share in 2025 due to continuous immunology research and academic advancements. Their broad scope of studies ranging from infectious disease immunology to tumor microenvironment analysis creates consistent demand for ELISpot assays. These centers often perform high-volume cytokine profiling and leverage ELISpot for exploratory and hypothesis-driven studies. Government-funded research programs and international collaborations further amplify usage across universities and national labs. Specialized immunology centers also drive innovation in assay development, reinforcing their leading role in ELISpot adoption. The diverse research needs across global institutes ensure sustained market dominance.
Biopharmaceutical companies are expected to grow at the fastest rate from 2026 to 2033, driven by expanding vaccine pipelines, immunotherapy trials, and biologics development. ELISpot assays are standard tools in preclinical and clinical trials to evaluate immune activation, treatment efficacy, and antigen-specific responses. As precision medicine advances, biopharma firms increasingly rely on ELISpot to generate regulatory-grade immune data supportive of FDA and EMA submissions. Growth is further propelled by rising investments in oncology, autoimmune therapies, and next-generation vaccines. As biopharma R&D intensifies globally, ELISpot adoption in industry settings is scaling rapidly.
Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Regional Analysis
- North America dominated the ELISpot market with the largest revenue share of 38.9% in 2025, supported by advanced biotechnology infrastructure, high R&D investments, and strong presence of leading assay developers, with the U.S. experiencing substantial growth in ELISpot utilization across vaccine pipelines, immunotherapy trials, and academic immunology programs
- Researchers and healthcare providers in the region highly value the sensitivity, reliability, and standardized protocols offered by ELISpot assays for evaluating T-cell and B-cell responses in vaccines, immunotherapies, and infectious disease studies
- This widespread adoption is further supported by strong government funding for biomedical research, high R&D expenditure by biopharmaceutical companies, and the presence of key ELISpot technology providers, establishing North America as the leading region for both diagnostic and research ELISpot applications
U.S. Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Insight
The U.S. Enzyme-Linked Immune Absorbent Spot (ELISpot) market captured the largest revenue share in North America in 2025, fueled by the rapid adoption of immune-monitoring technologies and strong investment in immunology research. Researchers and biopharmaceutical companies are prioritizing high-sensitivity assays to evaluate T-cell and B-cell responses in vaccines, cancer immunotherapies, and infectious disease studies. The growing preference for automated, high-throughput ELISpot systems, alongside integration with laboratory information management systems (LIMS), further propels market growth. Moreover, the U.S.’s robust healthcare infrastructure, well-funded research institutions, and presence of leading ELISpot technology providers significantly contribute to the market’s expansion.
Europe Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Insight
The Europe Enzyme-Linked Immune Absorbent Spot (ELISpot) market is projected to expand at a substantial CAGR during the forecast period, driven by increasing immunology research, stringent regulatory requirements for diagnostic assays, and rising adoption of standardized immune-monitoring techniques. Growing investments in vaccine development and translational research are fostering the use of ELISpot assays in hospitals, clinical labs, and research centers. European researchers also value the high sensitivity and reproducibility of ELISpot systems. The market is witnessing significant growth across both diagnostic and research applications, supported by collaborations between academic institutions and biopharmaceutical companies.
U.K. Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Insight
The U.K. Enzyme-Linked Immune Absorbent Spot (ELISpot) market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by increased focus on cellular immune-response monitoring in clinical trials and research initiatives. The rising prevalence of immuno-oncology studies and vaccine programs is encouraging both academic and industrial laboratories to adopt ELISpot assays. In addition, well-established laboratory infrastructure and strong funding support for immunology research are expected to stimulate market growth. The U.K.’s growing emphasis on precision medicine and high-throughput immune profiling further promotes the adoption of advanced ELISpot platforms.
Germany Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Insight
The Germany Enzyme-Linked Immune Absorbent Spot (ELISpot) market is expected to expand at a considerable CAGR during the forecast period, driven by heightened awareness of immune health, increasing adoption of advanced immunoassays, and government-backed research programs. Germany’s well-established biotechnology and pharmaceutical sector, combined with a strong focus on innovation and translational medicine, supports ELISpot adoption across research institutes and hospitals. Integration with automated analyzers and digital laboratory systems is becoming increasingly prevalent. There is also a strong preference for standardized, reproducible assays that comply with regulatory requirements, aligning with local research and clinical needs.
Asia-Pacific Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Insight
The Asia-Pacific Enzyme-Linked Immune Absorbent Spot (ELISpot) market is poised to grow at the fastest CAGR during the forecast period, fueled by expanding biomedical research, rising government funding for infectious disease and immunology programs, and increasing biopharmaceutical R&D activities in countries such as China, India, and Japan. The region’s growing focus on vaccine development, cancer research, and autoimmune disease studies is driving ELISpot adoption. Furthermore, improvements in laboratory infrastructure and increasing access to automated ELISpot systems are enhancing market penetration. Asia-Pacific is also emerging as a manufacturing hub for ELISpot reagents and platforms, improving affordability and accessibility.
Japan Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Insight
The Japan Enzyme-Linked Immune Absorbent Spot (ELISpot) market is gaining momentum due to the country’s advanced research ecosystem, strong focus on biotechnology, and rising immunology-driven studies. The market emphasizes highly sensitive and standardized immune assays for vaccine evaluation, infectious disease research, and oncology studies. Integration with laboratory automation systems and data management platforms is fueling growth. Moreover, increasing government and private funding for immunology research, coupled with rising adoption of high-throughput ELISpot platforms in academic and industrial laboratories, is driving market expansion.
India Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Insight
The India Enzyme-Linked Immune Absorbent Spot (ELISpot) market accounted for a significant revenue share in Asia-Pacific in 2025, attributed to the country’s growing biomedical research sector, increasing government and private investment in immunology, and rising demand for immune-monitoring solutions. India is becoming a key market for clinical and research applications of ELISpot, particularly in vaccine development, infectious disease studies, and immuno-oncology research. The expansion of laboratory infrastructure, availability of cost-effective ELISpot kits, and collaborations with international research organizations are key factors propelling market growth in India.
Enzyme-Linked Immune Absorbent Spot (ELISpot) Market Share
The Enzyme-Linked Immune Absorbent Spot (ELISpot) industry is primarily led by well-established companies, including:
- Cellular Technology Limited (U.S.)
- Oxford Immunotec (U.K.)
- Mabtech AB (Sweden)
- BD (U.S.)
- Abcam plc (U.K.)
- AID Autoimmun Diagnostika GmbH (Germany)
- U-CyTech (Netherlands)
- Full Moon BioSystems, Inc. (U.S.)
- Merck KGaA (Germany)
- Covalab (France)
- CEDARLANE (Canada)
- Thermo Fisher Scientific Inc. (U.S.)
- Creative Diagnostics (U.S.)
- StressMarq Biosciences Inc. (Canada)
- GeneTex, Inc. (U.S.)
- DIACLONE SAS (France)
- Bio-Techne (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- BioLegend, Inc. (U.S.)
- Revvity (U.K.)
What are the Recent Developments in Global Enzyme-Linked Immune Absorbent Spot (ELISpot) Market?
- In April 2025, Revvity secured FDA approval for its Auto‑Pure 2400 automated liquid handling platform integrated with the T‑SPOT.TB test, enabling high‑throughput ELISpot‑based latent tuberculosis testing by processing up to 24 samples per run in under 3.5 hours, reducing manual workload and improving diagnostic precision for clinical laboratories
- In January 2025, Mabtech launched the PepPool: CEFRAS Global family of positive control peptide pools for ELISpot and FluoroSpot assays, designed with a broad range of immunogenic epitopes to deliver robust T‑cell stimulation across diverse human populations, enhancing assay reliability and reproducibility in vaccine and immunotherapy research
- In November 2024, Mabtech expanded its immunoassay portfolio with the launch of new FluoroSpot Path: Human immunotherapy potency kits, enabling multiplex cytokine detection for detailed functional analysis of cell therapy products and supporting deeper immune profiling in research and development settings
- In April 2024, Oxford Immunotec launched an updated T‑SPOT Discovery SARS‑CoV‑2 ELISpot kit for research use, aimed at facilitating detailed study of cellular immune responses to COVID‑19, allowing researchers to measure antigen‑specific T‑cell reactivity beyond traditional antibody assays and support vaccine and immunological research
- In January 2021, Oxford Immunotec’s T‑SPOT Discovery SARS‑CoV‑2 test was used in a Phase I/II clinical study to assess T‑cell responses induced by Valneva’s VLA2001 COVID‑19 vaccine candidate, illustrating early application of ELISpot technology to gauge cellular immunity in vaccine evaluation studies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.