Global Evans Syndrome Market
Market Size in USD Billion
CAGR :
% 
 USD
80.50 Billion
USD
163.96 Billion
2024
2032
USD
80.50 Billion
USD
163.96 Billion
2024
2032
| 2025 –2032 | |
| USD 80.50 Billion | |
| USD 163.96 Billion | |
|
|
|
|
Evans Syndrome Market Size
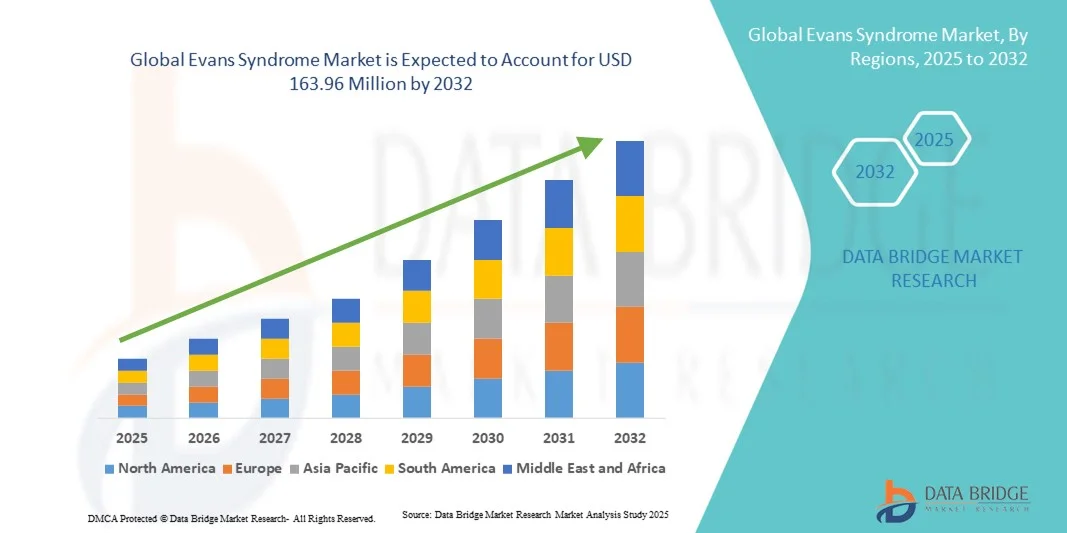
- The global Evans Syndrome market size was valued at USD 80.50 billion in 2024 and is expected to reach USD 163.96 million by 2032, at a CAGR of 9.30% during the forecast period
- The market growth is largely fueled by advancements in diagnostic techniques, therapeutic options, and increasing awareness of this rare autoimmune disorder, leading to better detection and management in both pediatric and adult populations
- Furthermore, rising patient demand for effective, targeted, and accessible treatment solutions is establishing improved immunosuppressive and biologic therapies as the modern standard of care. These converging factors are accelerating the adoption of Evans Syndrome therapies, thereby significantly boosting the industry's growth
Evans Syndrome Market Analysis
- Evans Syndrome, a rare autoimmune disorder characterized by simultaneous or sequential occurrence of autoimmune hemolytic anemia and immune thrombocytopenia, is increasingly recognized as a critical condition requiring specialized diagnosis and treatment in both pediatric and adult populations due to its complex and chronic nature
- The escalating demand for Evans Syndrome therapies is primarily fueled by advancements in diagnostic techniques, increasing awareness among healthcare professionals, and rising patient preference for targeted treatments such as corticosteroids, immunosuppressive drugs, and monoclonal antibodies that improve outcomes and quality of life
- North America dominated the Evans Syndrome market with the largest revenue share of 38.5% in 2024, driven by advanced healthcare infrastructure, early adoption of novel therapies, high healthcare expenditure, and active research and development initiatives, particularly in the U.S., where clinical trials and innovative treatment approaches are accelerating therapy adoption
- Asia-Pacific is expected to be the fastest growing region in the Evans Syndrome market during the forecast period due to increasing healthcare infrastructure, rising patient population, and growing accessibility to advanced diagnostic techniques such as blood tests, flow cytometry, and bone marrow biopsies
- Primary Evans Syndrome segment dominated the market with a share of 52.2% in 2024, driven by higher prevalence and the need for continuous monitoring, while hospitals remain the leading end-users due to concentrated treatment facilities and specialized care for complex case
Report Scope and Evans Syndrome Market Segmentation
|
Attributes |
Evans Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Evans Syndrome Market Trends
Advancements in Targeted Immunotherapies and Personalized Treatments
- A significant and accelerating trend in the global Evans Syndrome market is the increasing adoption of targeted immunotherapies and personalized treatment approaches, enhancing patient outcomes and quality of life
- For instance, monoclonal antibodies such as rituximab are being increasingly used alongside corticosteroids to manage refractory Evans Syndrome cases, offering more precise immune system modulation
- These therapies enable clinicians to reduce dependency on broad-spectrum immunosuppressive drugs while minimizing side effects and improving long-term disease management
- Furthermore, integration of genetic and molecular profiling in patient diagnosis allows for tailored treatment plans, improving the efficacy of therapies for both adults and children
- This trend towards personalized, targeted, and research-driven treatments is fundamentally reshaping patient expectations for care and management of Evans Syndrome
- The demand for advanced therapies and individualized treatment regimens is growing rapidly across both hospital and diagnostic settings, as patients and physicians increasingly prioritize effectiveness, safety, and minimized relapses
Evans Syndrome Market Dynamics
Driver
Increasing Awareness and Adoption of Advanced Diagnostic Techniques
- The growing awareness of Evans Syndrome among healthcare professionals and patients, combined with wider availability of advanced diagnostics, is a key driver for the rising demand for specialized treatment
- For instance, blood tests, flow cytometry, and bone marrow biopsies enable early and accurate detection, allowing timely intervention and improved patient prognosis
- As more clinicians recognize the complexity of Evans Syndrome and the need for proactive management, adoption of combination therapies such as corticosteroids with immunosuppressive drugs is increasing
- Furthermore, research initiatives and clinical trials are expanding treatment options, encouraging hospitals and diagnostic laboratories to adopt advanced care protocols
- The increasing focus on pediatric and adult patient populations, coupled with specialized hospital programs, is driving market growth in both developed and emerging regions
- Rising patient awareness and physician training programs on rare autoimmune disorders further boost early diagnosis and therapy adoption, reinforcing market expansion
Restraint/Challenge
High Treatment Costs and Limited Accessibility in Emerging Regions
- The high cost of advanced Evans Syndrome therapies, particularly biologics and combination treatments, poses a significant challenge to market growth
- For instance, monoclonal antibody therapies and stem cell transplantation remain expensive and often inaccessible in low- and middle-income regions, limiting widespread adoption
- Healthcare infrastructure gaps in emerging markets can delay diagnosis and treatment initiation, restricting timely access to effective therapies
- Furthermore, regulatory complexities for approval of new biologics and immunomodulatory drugs can slow market introduction, impacting patient access
- While awareness is increasing, limited healthcare coverage and high out-of-pocket expenses continue to hinder adoption of advanced treatment regimens
- Overcoming these challenges through improved healthcare accessibility, insurance coverage expansion, and cost-effective therapy development will be vital for sustained market growth
Evans Syndrome Market Scope
The market is segmented on the basis of type, diagnosis, treatment, population type, end-user, and distribution channel.
- By Type
On the basis of type, the Evans Syndrome market is segmented into primary evans syndrome and secondary evans syndrome. The Primary Evans Syndrome segment dominated the market with the largest revenue share of 52.2% in 2024, driven by its higher prevalence among autoimmune disorders with no known underlying condition. Patients with primary Evans Syndrome often require long-term management, including corticosteroids and immunosuppressive therapies, to control relapses and maintain stable blood counts. The dominance of this segment is further supported by increasing awareness and early diagnosis among clinicians, particularly in developed regions. Research initiatives and clinical trials focusing on primary Evans Syndrome are contributing to better treatment protocols, strengthening market adoption. The availability of personalized therapies and continuous monitoring solutions enhances patient compliance and outcome, sustaining the segment’s market leadership. Primary Evans Syndrome also receives greater funding and research attention compared to secondary cases, further boosting its market share.
The Secondary Evans Syndrome segment is anticipated to witness the fastest growth rate of 6.5% from 2025 to 2035, fueled by its association with other autoimmune conditions and malignancies, which increases the need for early detection and effective treatment. Rising prevalence of secondary cases due to conditions such as lupus and lymphoma is driving the adoption of combined therapeutic approaches. Clinicians are increasingly recognizing the benefits of targeted interventions such as monoclonal antibodies and IV immune globulin in secondary cases. Awareness programs and better diagnostic infrastructure in emerging markets are contributing to faster growth. The complexity of managing secondary Evans Syndrome also drives research into novel therapies and combination treatments, creating new market opportunities.
- By Diagnosis
On the basis of diagnosis, the Evans Syndrome market is segmented into blood test, bone marrow biopsy, flow cytometry, and CT Scans. The Blood Test segment dominated the market with the largest revenue share of 45% in 2024, driven by its routine use as the first-line diagnostic method for identifying autoimmune hemolytic anemia and thrombocytopenia. Blood tests are minimally invasive, widely accessible, and provide rapid results, making them essential in both pediatric and adult populations. Clinicians rely on blood tests for continuous monitoring of hemoglobin levels, platelet counts, and response to therapy. The segment’s dominance is also supported by increasing hospital and diagnostic lab infrastructure that prioritizes cost-effective and efficient screening methods. Blood tests are often complemented with advanced diagnostics for complex cases, reinforcing their central role. Continuous technological improvements in hematology analyzers are enhancing sensitivity and accuracy, further driving the segment.
The Flow Cytometry segment is expected to witness the fastest growth rate of 7.1% from 2025 to 2035, fueled by its ability to provide detailed immunophenotyping and accurate detection of autoimmune activity. Flow cytometry enables precise differentiation between primary and secondary Evans Syndrome, aiding personalized treatment planning. Its growing adoption in research institutes and specialty hospitals contributes to the segment’s growth. Increasing awareness of advanced diagnostic techniques and government support for rare disease detection further accelerates adoption. Flow cytometry’s capability to monitor treatment response and relapse risk also makes it an essential tool in modern Evans Syndrome management.
- By Treatment
On the basis of treatment, the Evans Syndrome market is segmented into corticosteroids, immunosuppressive drugs, monoclonal antibody, blood transfusion, IV immune globulin, and stem cell transplantation. The Corticosteroids segment dominated the market with the largest revenue share of 50% in 2024, driven by its role as the first-line therapy for both primary and secondary Evans Syndrome. Corticosteroids are widely prescribed due to their rapid effectiveness in reducing hemolysis and increasing platelet counts. Their dominance is supported by the large patient base and the relative ease of administration in both inpatient and outpatient settings. The segment benefits from extensive clinical experience and established treatment guidelines that make it a standard of care. Continuous research into dosing optimization and combination therapy with immunosuppressive drugs enhances patient outcomes. The affordability and availability of corticosteroids in most healthcare systems further reinforce their market leadership.
The Monoclonal Antibody segment is anticipated to witness the fastest growth rate of 8.2% from 2025 to 2035, fueled by its targeted mechanism of action against specific immune cells, offering improved efficacy for refractory and complex Evans Syndrome cases. Therapies such as rituximab are increasingly preferred for patients not responding to corticosteroids alone. Rising clinical trials, regulatory approvals, and insurance coverage in developed markets accelerate adoption. Monoclonal antibodies also reduce the need for long-term high-dose corticosteroids, minimizing side effects. Growing awareness among clinicians about personalized medicine approaches is driving market growth. Adoption in emerging markets is expected to increase as healthcare infrastructure and access to specialty treatments improve.
- By Population Type
On the basis of population type, the Evans Syndrome market is segmented into adults and children. The Adults segment dominated the market with the largest revenue share of 57% in 2024, driven by the higher incidence of Evans Syndrome in adults compared to pediatric populations. Adults often present with comorbidities that require integrated management, increasing demand for advanced therapies and continuous monitoring. Hospital-based treatment and diagnostic facilities are more readily available for adults, contributing to segment dominance. Clinical research and drug development primarily focus on adult patients, strengthening adoption of standard-of-care treatments. The segment’s dominance is also fueled by higher healthcare spending and insurance coverage in adult populations, particularly in North America and Europe. Rising awareness of autoimmune disorders in adults further supports continued market leadership.
The Children segment is expected to witness the fastest growth rate of 6.8% from 2025 to 2035, driven by increasing early diagnosis and better treatment protocols in pediatric care. Pediatric hospitals and specialty centers are adopting targeted therapies and supportive care solutions such as IV immune globulin and corticosteroids. Growing focus on rare pediatric autoimmune disorders and funding for research programs enhances adoption. Parents and caregivers are increasingly seeking advanced treatments to minimize complications and relapses in children. Technological advancements in pediatric monitoring and safer therapy options are also contributing to rapid growth.
- By End-User
On the basis of end-user, the Evans Syndrome market is segmented into research institutes, diagnostic laboratories, hospitals, and others. The Hospitals segment dominated the market with the largest revenue share of 60% in 2024, driven by the concentration of specialized care, treatment facilities, and multidisciplinary teams managing Evans Syndrome. Hospitals provide both acute care and long-term management, making them the primary point of treatment for patients. Advanced diagnostic tools, therapeutic administration, and follow-up services are more accessible in hospital settings, reinforcing segment dominance. Hospitals also participate in clinical trials, contributing to treatment adoption and research. The availability of trained clinicians and comprehensive patient monitoring supports continuous care. Collaboration with diagnostic laboratories further enhances hospitals’ role as the dominant end-user.
The Diagnostic Laboratories segment is expected to witness the fastest growth rate of 7.5% from 2025 to 2035, fueled by the rising need for accurate and early detection using blood tests, flow cytometry, and bone marrow biopsies. Specialized labs offer high-precision diagnostics that complement hospital treatments. Increasing awareness among physicians and patients about advanced diagnostic options drives growth. Laboratory expansion in emerging markets improves access to testing and monitoring. Technological advancements and automated testing solutions enhance efficiency and reliability. Partnerships between diagnostic labs and hospitals further strengthen market opportunities.
- By Distribution Channel
On the basis of distribution channel, the Evans Syndrome market is segmented into hospital pharmacy, retail pharmacy, and others. The Hospital Pharmacy segment dominated the market with the largest revenue share of 62% in 2024, driven by the centralized procurement of therapies and administration of specialized treatments such as IV immune globulin and monoclonal antibodies within hospital settings. Hospital pharmacies provide controlled dosing, patient counseling, and close monitoring of adverse effects. Their dominance is reinforced by the direct link to physicians prescribing treatment and the ability to manage complex combination therapies. Hospital pharmacies also facilitate participation in clinical trials and access to newly approved therapies. The segment benefits from healthcare infrastructure and reimbursement policies that favor hospital-based treatment. Efficient inventory management and cold chain logistics further strengthen dominance.
The Retail Pharmacy segment is expected to witness the fastest growth rate of 6.9% from 2025 to 2035, fueled by increasing patient access to outpatient therapies and supportive care medications. Retail pharmacies provide convenience for ongoing prescriptions such as corticosteroids and immunosuppressive drugs. Expansion of retail chains in urban and semi-urban regions improves reach. Awareness campaigns and collaboration with clinicians help drive patient adoption. Growth is supported by regulatory frameworks allowing specialty medications in retail settings. The segment also benefits from e-pharmacy platforms enhancing delivery and accessibility.
Evans Syndrome Market Regional Analysis
- North America dominated the Evans Syndrome market with the largest revenue share of 38.5% in 2024, driven by advanced healthcare infrastructure, early adoption of novel therapies, high healthcare expenditure, and active research and development initiatives
- Patients and healthcare providers in the region prioritize timely diagnosis, access to advanced treatments such as monoclonal antibodies and IV immune globulin, and comprehensive monitoring programs for both adults and children
- This widespread adoption is further supported by well-established hospitals, research institutes, and diagnostic laboratories, along with high healthcare expenditure and strong insurance coverage, establishing North America as a leading region for Evans Syndrome management
U.S. Evans Syndrome Market Insight
The U.S. Evans Syndrome market captured the largest revenue share of 41% in 2024 within North America, fueled by advanced healthcare infrastructure, early adoption of novel therapies, and high awareness of rare autoimmune disorders. Patients increasingly prioritize timely diagnosis and access to advanced treatments such as monoclonal antibodies, IV immune globulin, and combination immunosuppressive therapies. The growing presence of specialized hospitals, research institutes, and diagnostic laboratories further propels the market. Moreover, ongoing clinical trials, patient support programs, and increasing insurance coverage for rare disease management are significantly contributing to market expansion.
Europe Evans Syndrome Market Insight
The Europe Evans Syndrome market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by well-established healthcare systems, increasing clinical research activities, and government initiatives supporting rare disease management. Rising awareness of autoimmune disorders and adoption of advanced diagnostics such as flow cytometry and bone marrow biopsies foster early detection. The market growth is also fueled by hospitals and specialty centers incorporating targeted therapies, while collaborations between research institutes and pharmaceutical companies enhance treatment availability across the region.
U.K. Evans Syndrome Market Insight
The U.K. Evans Syndrome market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing awareness of rare autoimmune diseases and growing adoption of advanced treatment protocols. Rising demand for early diagnosis and targeted therapies, alongside the presence of specialty hospitals and diagnostic laboratories, is encouraging better disease management. In addition, healthcare initiatives, patient advocacy programs, and strong regulatory support for rare disease treatments are expected to continue stimulating market growth.
Germany Evans Syndrome Market Insight
The Germany Evans Syndrome market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of autoimmune disorders, advanced healthcare infrastructure, and the availability of novel treatment options. Hospitals and research centers are increasingly adopting targeted therapies and personalized management plans. Germany’s emphasis on innovation, high standards of healthcare, and collaborations between hospitals and diagnostic laboratories promote the adoption of advanced diagnostic and therapeutic solutions, particularly for complex cases.
Asia-Pacific Evans Syndrome Market Insight
The Asia-Pacific Evans Syndrome market is poised to grow at the fastest CAGR of 7% during the forecast period, driven by improving healthcare infrastructure, rising awareness of rare autoimmune diseases, and increasing access to advanced diagnostics and therapies in countries such as China, India, and Japan. Government initiatives supporting rare disease management and increasing hospital specialization in autoimmune disorders are driving market adoption. Furthermore, growing patient populations and rising disposable incomes enhance the accessibility and affordability of therapies, expanding the treatment base across the region.
Japan Evans Syndrome Market Insight
The Japan Evans Syndrome market is gaining momentum due to the country’s advanced healthcare system, high awareness of autoimmune disorders, and increasing demand for effective treatment protocols. Hospitals and specialty centers focus on early diagnosis, targeted therapy, and continuous monitoring, driving adoption of therapies such as corticosteroids, immunosuppressive drugs, and monoclonal antibodies. In addition, ongoing clinical research, patient support programs, and collaborations between hospitals and diagnostic laboratories are further fueling market growth in both pediatric and adult populations.
India Evans Syndrome Market Insight
The India Evans Syndrome market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to improving healthcare infrastructure, rising awareness of rare autoimmune disorders, and increasing access to advanced diagnostics and therapies. Specialized hospitals and diagnostic laboratories are expanding in urban areas, while government initiatives promoting rare disease management support patient access. The growing middle class, rising healthcare expenditure, and adoption of modern therapies such as IV immune globulin and monoclonal antibodies are key factors propelling market growth in India.
Evans Syndrome Market Share
The Evans Syndrome industry is primarily led by well-established companies, including:
- AbbVie Inc. (U.S.)
- Alexion Pharmaceuticals, Inc. (U.S.)
- Amgen Inc. (U.S.)
- Astellas Pharma (Japan)
- Bayer AG (Germany)
- BD (U.S.)
- Biocryst Pharmaceuticals, Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Catalyst Pharmaceuticals, Inc. (U.S.)
- ChemoMetec A/S (Denmark)
- Chugai Pharmaceutical Co., Ltd. (Japan)
- Enzon Pharmaceuticals, Inc. (U.S.)
- Eli Lilly and Company (U.S.)
- Gilead Sciences, Inc. (U.S.)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- Takeda Pharmaceutical Company Limited (Japan)
What are the Recent Developments in Global Evans Syndrome Market?
- In September 2025, Dr. Illiasul Ibad, a rheumatologist at Christian Medical College, published new clinical practice guidelines for Evans Syndrome. These guidelines emphasize the need for structured diagnosis, tailored immunosuppression, and thoughtful supportive care, moving beyond the traditional view of Evans Syndrome as merely a combination of immune thrombocytopenia (ITP) and autoimmune hemolytic anemia (AIHA)
- In June 2025, a consensus developed by the Red Blood Cell Diseases (Anemia) Group under the Chinese Society of Hematology within the Chinese Medical Association was published. This consensus aims to standardize the diagnosis and management of Evans Syndrome in China, addressing the lack of prospective evidence and randomized clinical trials for the disease in the country
- In March 2025, a study published in Internal Medicine reported that Rituximab, a monoclonal antibody, was effective in treating new-onset Evans Syndrome in patients also diagnosed with systemic lupus erythematosus (SLE) and lupus nephritis. This finding suggests that Rituximab may be a viable treatment option for patients with newly diagnosed Evans Syndrome, expanding therapeutic possibilities
- In October 2024, Dr. Emily Harris, a clinical fellow in pediatric hematology/oncology at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, received the Michael Blaese Research Grant Award. The grant, funded by the Immune Deficiency Foundation, supports her proposal to investigate Evans Syndrome, aiming to develop better diagnostic and treatment tools for this rare autoimmune disorder
- In July 2024, a pilot study published in PubMed reported that three patients with refractory autoimmune hemolytic anemia (AIHA) and Evans Syndrome achieved complete or partial remission after treatment with Orelabrutinib, a Bruton's tyrosine kinase inhibitor. This suggests that Orelabrutinib could become a new second-line treatment for relapsed/refractory AIHA and Evans Syndrome
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.