Global Fallopian Tube Cancer Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.80 Billion
USD
3.35 Billion
2025
2033
USD
1.80 Billion
USD
3.35 Billion
2025
2033
| 2026 –2033 | |
| USD 1.80 Billion | |
| USD 3.35 Billion | |
|
|
|
|
Fallopian Tube Cancer Treatment Market Size
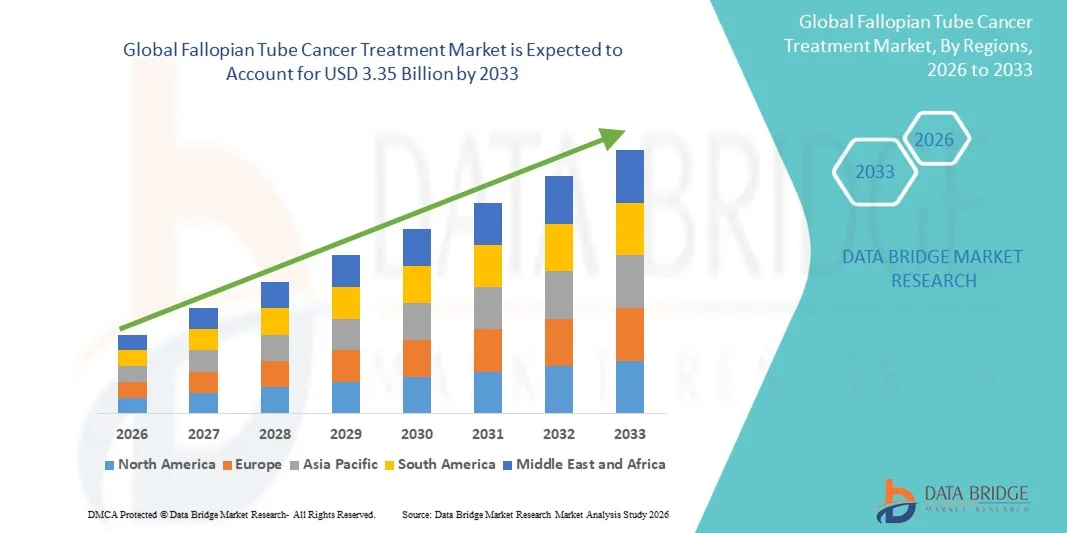
- The global fallopian tube cancer treatment market size was valued at USD 1.80 billion in 2025 and is expected to reach USD 3.35 billion by 2033, at a CAGR of 8.10% during the forecast period
- The market growth is largely driven by increasing awareness of gynecological cancers, rising incidence of fallopian tube cancer, and continuous advancements in diagnostic technologies, leading to earlier detection and improved treatment outcomes across both developed and emerging healthcare systems
- Furthermore, growing adoption of advanced treatment approaches such as targeted therapies, chemotherapy combinations, and minimally invasive surgical techniques, along with expanding access to specialized oncology care, is accelerating the uptake of fallopian tube cancer treatment solutions, thereby significantly boosting the industry’s growth
Fallopian Tube Cancer Treatment Market Analysis
- The Fallopian Tube Cancer Treatment market is driven by rising incidence of gynecological cancers, increasing awareness of rare ovarian and fallopian tube malignancies, and continuous advancements in early diagnostic techniques, including imaging and biomarker-based screening, across both developed and emerging healthcare systems
- Furthermore, growing adoption of advanced treatment modalities such as combination chemotherapy, targeted therapy, immunotherapy, and minimally invasive surgical procedures is improving patient outcomes and expanding treatment accessibility, thereby significantly accelerating market growth
- North America dominated the fallopian tube cancer treatment market with the largest revenue share of 39.2% in 2025, supported by advanced oncology infrastructure, high healthcare expenditure, strong presence of leading pharmaceutical companies, and widespread availability of innovative treatment options. The U.S. led the region with substantial investments in clinical trials and adoption of novel therapies
- Asia-Pacific is expected to be the fastest-growing region, registering a CAGR of 8.5% during the forecast period, driven by increasing cancer diagnosis rates, improving healthcare infrastructure, expanding access to oncology treatments, and rising government initiatives focused on women’s health and cancer care
- The Serous Adenocarcinomas segment dominated the largest market revenue share of 64.8% in 2025, primarily due to its high prevalence among diagnosed fallopian tube cancer cases
Report Scope and Fallopian Tube Cancer Treatment Market Segmentation
|
Attributes |
Fallopian Tube Cancer Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Fallopian Tube Cancer Treatment Market Trends
“Advancements in Targeted Therapies and Personalized Treatment Approaches”
- A significant and accelerating trend in the global fallopian tube cancer treatment market is the increasing adoption of targeted therapies and personalized medicine approaches. Advances in molecular biology and genetic profiling are enabling clinicians to tailor treatment strategies based on individual tumor characteristics, improving treatment efficacy and patient outcomes
- The growing use of PARP inhibitors and platinum-based chemotherapy regimens is reshaping treatment protocols, particularly for patients with BRCA mutations
- For instance, drugs such as olaparib and niraparib are increasingly being incorporated into maintenance therapy regimens following chemotherapy, demonstrating improved progression-free survival in gynecologic cancers, including fallopian tube cancer
- Another key trend is the rising integration of immunotherapy into treatment pipelines. Ongoing clinical trials are evaluating immune checkpoint inhibitors in combination with chemotherapy, expanding therapeutic options for patients with advanced or recurrent disease
- The increasing focus on early-stage diagnosis through improved imaging technologies and biomarker testing is contributing to better treatment planning and improved survival rates. Early detection allows for more effective surgical intervention followed by adjuvant therapies
- In addition, growing collaboration between pharmaceutical companies, research institutions, and oncology centers is accelerating drug development and expanding access to innovative therapies across developed and emerging markets
Fallopian Tube Cancer Treatment Market Dynamics
Driver
“Rising Incidence of Gynecologic Cancers and Improved Diagnostic Capabilities”
- The increasing incidence of gynecologic cancers, including fallopian tube cancer, is a primary driver for market growth. Greater awareness of hereditary cancer syndromes and improved screening for high-risk populations are leading to higher diagnosis rates globally
- Advancements in diagnostic imaging and genetic testing are enabling more accurate and earlier detection of fallopian tube cancer
- For instance, the growing use of transvaginal ultrasound, MRI, and BRCA genetic testing in oncology centers has significantly improved diagnostic precision and treatment decision-making
- Expanding healthcare infrastructure and increasing access to specialized oncology services in emerging economies are further supporting market expansion. Governments and private healthcare providers are investing in cancer treatment facilities and advanced therapeutics
- The growing availability of novel drugs and combination therapies is improving survival outcomes, encouraging wider adoption of advanced treatment regimens among clinicians
- In addition, rising healthcare expenditure and favorable reimbursement policies for oncology drugs in several developed regions are facilitating patient access to advanced fallopian tube cancer treatments
Restraint/Challenge
“High Treatment Costs and Limited Disease Awareness”
- The high cost associated with advanced cancer therapies, including targeted drugs and immunotherapies, remains a significant challenge for the Fallopian Tube Cancer Treatment market
- These therapies can impose a substantial financial burden on healthcare systems and patients, particularly in low- and middle-income countries
- Limited awareness of fallopian tube cancer due to its rarity often leads to delayed diagnosis and treatment
- For instance, symptoms are frequently misattributed to ovarian or general gynecologic conditions, resulting in late-stage detection and reduced treatment effectiveness
- The lack of disease-specific clinical guidelines in some regions can lead to variability in treatment approaches, affecting consistency in patient outcomes
- Adverse effects associated with chemotherapy and targeted therapies, including toxicity and long-term complications, may limit treatment adherence and patient tolerance
- Overcoming these challenges will require increased awareness initiatives, improved access to affordable therapies, expanded clinical research, and supportive healthcare policies to ensure sustained market growth
Fallopian Tube Cancer Treatment Market Scope
The market is segmented on the basis of type, treatment type, and end user.
• By Type
On the basis of type, the Global Fallopian Tube Cancer Treatment market is segmented into Serous Adenocarcinomas and Endometrioid Adenocarcinomas. The Serous Adenocarcinomas segment dominated the largest market revenue share of 64.8% in 2025, primarily due to its high prevalence among diagnosed fallopian tube cancer cases. Serous adenocarcinomas are considered the most aggressive and common histological subtype, often detected at advanced stages. Their clinical similarity to ovarian cancer leads to higher diagnostic frequency and treatment intervention. The segment benefits from extensive clinical research and established treatment protocols. Higher chemotherapy responsiveness supports treatment adoption. Increased awareness among oncologists improves early identification. Strong inclusion in clinical trials accelerates therapy development. Advanced imaging and biomarker-based diagnostics aid detection. Higher mortality risk necessitates aggressive treatment approaches. Availability of targeted therapies further strengthens market dominance. Ongoing pharmaceutical investments reinforce segment leadership.
The Endometrioid Adenocarcinomas segment is expected to witness the fastest CAGR of 7.9% from 2026 to 2033, driven by improved diagnostic accuracy and growing awareness of rare gynecologic cancers. Increasing use of molecular pathology and genetic profiling supports early-stage detection. These tumors are often associated with better prognosis, encouraging proactive treatment. Advancements in personalized medicine enhance therapeutic outcomes. Rising adoption of hormone-based therapies fuels growth. Expansion of specialized oncology centers improves access to care. Increased research focus on rare cancers boosts funding and innovation. Improved patient survival rates encourage long-term therapy utilization. Growing screening programs contribute to diagnosis rates. These factors collectively support accelerated growth during the forecast period.
• By Treatment Type
On the basis of treatment type, the Global Fallopian Tube Cancer Treatment market is segmented into Surgery, Chemotherapy, Targeted Therapy, Hormone Therapy, and Adjuvant (Targeted) Therapy. The Chemotherapy segment accounted for the largest market revenue share of 41.6% in 2025, driven by its central role as a first-line and adjuvant treatment. Platinum-based chemotherapy remains the standard of care for fallopian tube cancer. High efficacy in reducing tumor burden supports widespread adoption. Chemotherapy is commonly used post-surgery to prevent recurrence. Broad availability across healthcare settings strengthens usage. Established reimbursement policies improve patient access. Continuous development of combination regimens enhances outcomes. Increasing global cancer incidence sustains demand. Chemotherapy’s applicability across disease stages supports dominance. High clinician familiarity ensures consistent utilization. Ongoing improvements in drug formulations reduce side effects and improve compliance.
The Targeted Therapy segment is anticipated to witness the fastest CAGR of 9.3% from 2026 to 2033, fueled by advances in precision oncology. Targeted drugs offer improved efficacy with fewer systemic side effects. Growing adoption of PARP inhibitors and anti-angiogenic agents supports growth. Increased genetic testing enables patient-specific treatment selection. Favorable clinical trial outcomes accelerate regulatory approvals. Rising demand for personalized cancer care boosts adoption. Improved patient survival rates drive long-term therapy use. Expanding pipelines from pharmaceutical companies strengthen availability. Increasing awareness among oncologists promotes usage. These factors collectively contribute to robust growth momentum.
• By End User
On the basis of end user, the Global Fallopian Tube Cancer Treatment market is segmented into Hospitals, Diagnostic Centers, Home Healthcare, and Others. The Hospitals segment dominated the market with a revenue share of 58.9% in 2025, driven by the complexity of fallopian tube cancer management. Hospitals serve as primary centers for diagnosis, surgery, and chemotherapy administration. Availability of multidisciplinary oncology teams supports comprehensive care. Advanced surgical infrastructure enhances treatment outcomes. Hospitals are major sites for clinical trials and advanced therapies. High patient inflow supports continuous demand. Integration of diagnostic imaging and pathology services strengthens dominance. Government and private investments in hospital oncology units further boost capacity. Higher trust among patients encourages hospital-based treatment. Reimbursement coverage is stronger in hospital settings, reinforcing leadership.
The Home Healthcare segment is expected to register the fastest CAGR of 8.5% from 2026 to 2033, driven by the growing shift toward outpatient and supportive cancer care. Advances in oral chemotherapy and targeted drugs support home administration. Increasing emphasis on patient comfort and quality of life fuels demand. Cost-effectiveness compared to inpatient care encourages adoption. Technological advancements enable remote monitoring and tele-oncology. Aging populations increase demand for home-based care. Improved caregiver training enhances treatment safety. Rising healthcare expenditure pressures support decentralized care models. Expansion of home infusion services strengthens growth. These factors collectively drive rapid expansion of this segment.
Fallopian Tube Cancer Treatment Market Regional Analysis
- North America dominated the fallopian tube cancer treatment market with the largest revenue share of 39.2% in 2025, supported by advanced oncology infrastructure, high healthcare expenditure, strong presence of leading pharmaceutical companies, and widespread availability of innovative treatment options
- The market accounted for a major share of regional demand, driven by substantial investments in clinical trials, early adoption of targeted therapies, immunotherapies, and combination treatment regimens
- The region also benefits from well-established reimbursement policies, access to cutting-edge diagnostic tools, and high patient awareness, all contributing to consistent growth in the Fallopian Tube Cancer Treatment market
U.S. Fallopian Tube Cancer Treatment Market Insight
The U.S. fallopian tube cancer treatment market captured the largest revenue share in North America in 2025. Growth is fueled by increasing investments in R&D for novel therapeutics such as PARP inhibitors, targeted monoclonal antibodies, and combination chemotherapy regimens. Leading pharmaceutical companies are expanding clinical trials to explore personalized treatment protocols. Moreover, the presence of advanced cancer treatment centers, early diagnostic facilities, and high adoption of minimally invasive surgical techniques are further boosting market demand.
Europe Fallopian Tube Cancer Treatment Market Insight
The Europe fallopian tube cancer treatment market is projected to expand at a substantial CAGR during the forecast period, driven by increasing awareness of women’s health issues, government support for oncology research, and rising adoption of advanced therapeutics. Key countries such as the UK, Germany, and France are witnessing growth due to improved early diagnosis programs, widespread clinical trial participation, and accessibility of innovative treatment options including immunotherapies and targeted drug delivery systems.
U.K. Fallopian Tube Cancer Treatment Market Insight
The U.K. fallopian tube cancer treatment market is anticipated to grow at a noteworthy CAGR, fueled by increasing investments in cancer research, government-backed screening programs, and rising adoption of precision medicines. Early detection initiatives and public awareness campaigns are driving patient participation in advanced treatment protocols, further supporting market expansion.
Germany Fallopian Tube Cancer Treatment Market Insight
Germany’s fallopian tube cancer treatment market is expected to expand at a considerable CAGR, driven by strong healthcare infrastructure, high prevalence of gynecological cancers, and early adoption of novel therapies. Investments in oncology-focused R&D, combined with widespread access to specialized treatment centers, are key factors enhancing the market’s growth.
Asia-Pacific Fallopian Tube Cancer Treatment Market Insight
The Asia-Pacific fallopian tube cancer treatment market is poised to grow at the fastest CAGR of 8.5% during the forecast period, supported by increasing cancer diagnosis rates, improving healthcare infrastructure, and expanding access to oncology treatments. Government initiatives focused on women’s health, rising awareness of early diagnosis, and growing adoption of targeted and combination therapies are accelerating market growth across countries such as China, India, Japan, and South Korea.
Japan Fallopian Tube Cancer Treatment Market Insight
The Japan fallopian tube cancer treatment market is witnessing growth due to its high healthcare standards, strong government support for oncology research, and early adoption of innovative therapies. The country’s aging population and increased focus on women’s health are further driving demand for advanced treatment options and specialized cancer care centers.
China Fallopian Tube Cancer Treatment Market Insight
China fallopian tube cancer treatment market accounted for the largest market share in Asia-Pacific in 2025. The market growth is attributed to expanding oncology infrastructure, increasing prevalence of gynecological cancers, rising awareness of early treatment, and strong government initiatives promoting access to modern therapies. Local pharmaceutical investments and clinical trial expansion for targeted therapies are also contributing to the market’s rapid growth.
Fallopian Tube Cancer Treatment Market Share
The Fallopian Tube Cancer Treatment industry is primarily led by well-established companies, including:
- Roche (Switzerland)
- Novartis (Switzerland)
- AstraZeneca (UK)
- Pfizer (U.S.)
- Bristol-Myers Squibb (U.S.)
- Johnson & Johnson (U.S.)
- Merck & Co. (U.S.)
- GlaxoSmithKline (UK)
- Eli Lilly and Company (U.S.)
- Takeda Pharmaceutical (Japan)
- Bayer (Germany)
- AbbVie (U.S.)
- Sanofi (France)
- Amgen (U.S.)
- BeiGene (China)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Seattle Genetics (U.S.)
- OncoMed Pharmaceuticals (U.S.)
- Clovis Oncology (U.S.)
Latest Developments in Global Fallopian Tube Cancer Treatment Market
- In April 2021, BeiGene Ltd. announced that pamiparib (brand name Partruvix), a selective PARP‑1/2 inhibitor, received its first global registration in China for the treatment of women with recurrent ovarian, fallopian tube, or primary peritoneal cancer associated with germline BRCA mutations, offering a targeted therapy option for patients with DNA repair deficiencies
- In November 2022, the U.S. Food and Drug Administration (FDA) granted accelerated approval to mirvetuximab soravtansine‑gynx (Elahere) — a first‑in‑class folate receptor alpha (FRα)‑directed antibody‑drug conjugate — for adults with FRα‑positive, platinum‑resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer after prior systemic therapies, expanding targeted treatment options for resistant disease
- In March 2024, the FDA converted the accelerated approval of mirvetuximab soravtansine (Elahere) to a full approval for use in FRα‑positive, platinum‑resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer, reflecting robust clinical data and making it a standard treatment for these indications
- In January 2025, IMPACT Therapeutics announced that the National Medical Products Administration (NMPA) in China approved senaparib Capsules (派舒宁), a novel PARP inhibitor, for maintenance treatment in adult patients with advanced high‑grade ovarian, fallopian tube, or primary peritoneal cancer following first‑line platinum‑based chemotherapy, offering an additional targeted maintenance therapy option
- In January 2025, Zentalis Pharmaceuticals Inc. disclosed that azenosertib, a WEE1 kinase inhibitor, received FDA Fast Track designation for treating patients with platinum‑resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer who are positive for cyclin E1 by immunohistochemistry, potentially expediting its clinical development and regulatory review for these cancers
- In March 2025, AstraZeneca and Daiichi Sankyo entered a strategic collaboration to co‑develop and co‑promote olaparib outside the United States, strengthening efforts to expand access to PARP inhibitor therapy for fallopian tube and related cancers in global markets
- In May 2025, the U.S. FDA granted accelerated approval to Zejula (niraparib), a PARP inhibitor developed by AstraZeneca and Merck KGaA, for the treatment of recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer, representing a significant regulatory milestone in expanding maintenance therapy options
- In April 2025, Biocon Biologics received FDA approval for Jobevne (bevacizumab‑nwgd), a biosimilar to Avastin, which is indicated for multiple cancers including ovarian, fallopian tube, and primary peritoneal cancer, increasing access to anti‑angiogenic therapy globally
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.