Global Fluoroquinolone Toxicity Syndrome Market
Market Size in USD Billion
CAGR :
% 
 USD
1.49 Billion
USD
2.32 Billion
2024
2032
USD
1.49 Billion
USD
2.32 Billion
2024
2032
| 2025 –2032 | |
| USD 1.49 Billion | |
| USD 2.32 Billion | |
|
|
|
|
Fluoroquinolone Toxicity Syndrome Market Size
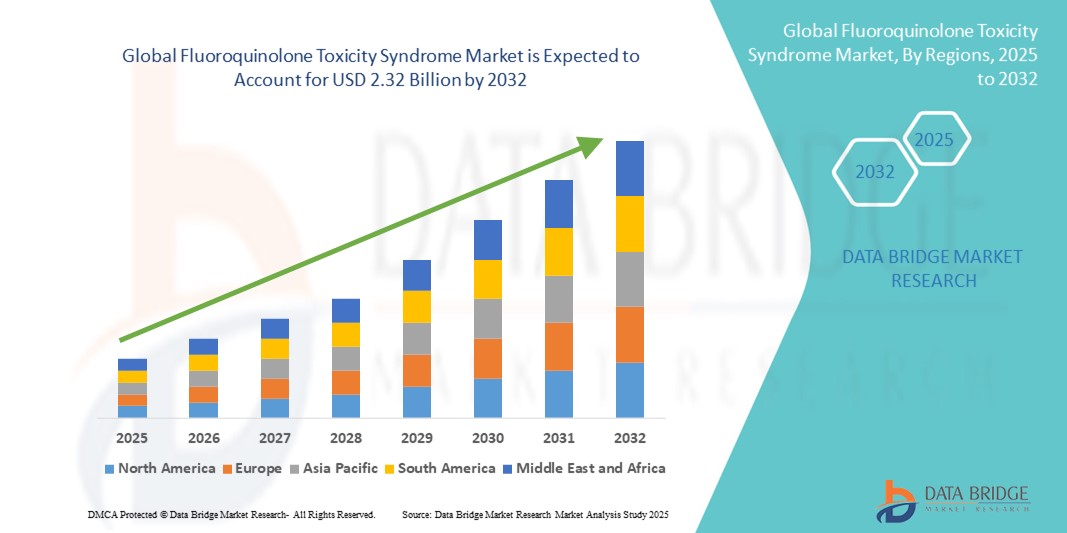
- The global fluoroquinolone toxicity syndrome market size was valued at USD 1.49 billion in 2024 and is expected to reach USD 2.32 billion by 2032, at a CAGR of 5.70% during the forecast period
- The market growth is largely fueled by increasing awareness of the adverse effects associated with fluoroquinolone antibiotics and the rising emphasis on early detection and management of Fluoroquinolone Toxicity Syndrome
- Furthermore, growing research initiatives, technological advancements in diagnostic tools, and the development of novel therapeutic approaches are contributing to the rapid adoption of effective treatment solutions. These converging factors are accelerating the uptake of fluoroquinolone toxicity syndrome management solutions, thereby significantly boosting the industry's growth
Fluoroquinolone Toxicity Syndrome Market Analysis
- The Fluoroquinolone Toxicity Syndrome market is witnessing significant growth due to rising awareness of drug-related adverse effects, increasing prevalence of bacterial infections treated with fluoroquinolones, and expanding healthcare infrastructure across both developed and emerging regions
- The escalating demand for effective monitoring and management of fluoroquinolone-related toxicity is primarily fueled by the increasing adoption of pharmacovigilance programs, growing awareness among healthcare professionals, and the need for safer therapeutic alternatives
- North America dominated the fluoroquinolone toxicity syndrome market with the largest revenue share of 40.5% in 2024, supported by advanced healthcare infrastructure, widespread adoption of diagnostic and monitoring services, and strong presence of pharmaceutical companies investing in safer therapeutic alternatives. The U.S. remains the major contributor, driven by rising awareness campaigns on fluoroquinolone-associated risks, proactive pharmacovigilance programs, and early intervention strategies that enhance market demand
- Asia-Pacific is expected to be the fastest-growing region in the fluoroquinolone toxicity syndrome market during the forecast period, with a CAGR of 9.1% from 2025 to 2032. Growth is fueled by increasing urbanization, rising disposable incomes, expansion of healthcare facilities offering specialized infectious disease management, and growing awareness among patients and physicians regarding adverse drug reactions related to fluoroquinolone
- The Age segment dominated the fluoroquinolone toxicity syndrome market with a share of 47.0% in 2024, driven by higher susceptibility among older patients to adverse effects of fluoroquinolones
Report Scope and Fluoroquinolone Toxicity Syndrome Market Segmentation
|
Attributes |
Fluoroquinolone Toxicity Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Fluoroquinolone Toxicity Syndrome Market Trends
Focus on Early Detection, Risk Management, and Patient Safety
- A notable trend in the global fluoroquinolone toxicity syndrome market is the increasing focus on early detection, risk management, and patient safety protocols. This emphasis is enhancing the effectiveness of treatment regimens and reducing the incidence of severe adverse effects associated with fluoroquinolone use
- Pharmaceutical companies and healthcare providers are investing in comprehensive pharmacovigilance programs, real-world evidence studies, and clinical monitoring to better understand patient responses. These initiatives are helping identify high-risk populations, optimize dosage guidelines, and support safer prescribing practices
- Regulatory agencies in key regions are strengthening guidelines for fluoroquinolone prescription and monitoring, which is prompting healthcare institutions to adopt standardized screening and reporting mechanisms. This ensures timely intervention and minimizes long-term complications
- The rising adoption of supportive therapies and adjunctive care measures, such as antioxidants, hydration management, and liver/kidney function monitoring, is further driving market growth. These measures help mitigate toxicity risks and improve patient outcomes during fluoroquinolone treatment
- Increasing awareness among clinicians and patients regarding the potential side effects of fluoroquinolones is creating higher demand for diagnostic tests, monitoring solutions, and patient education programs. Such awareness campaigns contribute to safer therapeutic use and reduce litigation risks
- Overall, the trend toward comprehensive management, early detection, and patient-centered care is significantly shaping the Fluoroquinolone Toxicity Syndrome market, with stakeholders prioritizing safety, efficacy, and regulatory compliance across both hospital and outpatient settings
Fluoroquinolone Toxicity Syndrome Market Dynamics
Driver
Growing Need Due to Rising Incidence and Awareness of Fluoroquinolone Toxicity
- The increasing prevalence of adverse effects associated with fluoroquinolone antibiotics, including tendon damage, neuropathy, and cardiotoxicity, is driving heightened awareness and demand for effective diagnostic and treatment solutions for fluoroquinolone toxicity syndrome
- For instance: In 2024, multiple pharmaceutical companies and research institutions initiated clinical programs and awareness campaigns to improve early detection, risk assessment, and management of fluoroquinolone-related toxicities. These proactive strategies by key stakeholders are expected to significantly propel market growth in the forecast period
- Clinicians are increasingly prioritizing patient safety and proactive monitoring, leading to a rising demand for innovative therapies, diagnostic tools, and patient-specific management protocols that reduce the risk of adverse drug reaction
- The adoption of personalized medicine approaches, coupled with patient-centric healthcare models, is enhancing the focus on individualized treatment plans, ensuring better outcomes for patients affected by fluoroquinolone toxicity
- Investments in biomarker research, pharmacovigilance programs, and advanced monitoring technologies are strengthening the ability of healthcare providers to detect early signs of toxicity and intervene promptly
- Government initiatives, regulatory guidance, and clinical guidelines emphasizing safe antibiotic use, early intervention, and post-treatment monitoring are further boosting adoption of therapeutic and diagnostic solutions, ensuring improved management of fluoroquinolone toxicity syndrome
Restraint/Challenge
Concerns Regarding Treatment Complexity and Cost of Management
- Managing fluoroquinolone toxicity syndrome can be highly complex due to the variability in patient reactions, presence of comorbidities, and the need for multidisciplinary interventions, which limits broader market adoption
- High costs associated with specialized diagnostic tests, monitoring tools, and advanced therapeutic interventions pose significant challenges, particularly for smaller clinics or budget-constrained healthcare providers in emerging markets
- Addressing these obstacles requires development of cost-effective treatment solutions, simplified diagnostic protocols, and training programs for healthcare professionals to enhance confidence and accessibility in clinical practice
- Limited awareness among patients and healthcare providers in certain regions, along with inconsistent availability of standardized treatment guidelines, can delay early detection and effective management of fluoroquinolone-related adverse events
- Despite growing adoption of newer interventions, the high infrastructure requirements and expenses associated with advanced therapies can hinder widespread utilization, especially in resource-limited settings
- Overcoming these challenges through affordable and scalable therapies, enhanced healthcare professional education, and the establishment of standardized clinical protocols will be critical for sustaining long-term growth of the Fluoroquinolone Toxicity Syndrome market
Fluoroquinolone Toxicity Syndrome Market Scope
The market is segmented on the basis of symptoms, diagnosis method, treatment approach, risk factors, end-users, and distribution channel.
- By Symptoms
On the basis of symptoms, the fluoroquinolone toxicity syndrome market is segmented into tendinopathy, peripheral neuropathy, CNS effects, and cardiovascular issues. The Tendinopathy segment dominated the largest market revenue share of 45.2% in 2024, owing to its high prevalence among patients using fluoroquinolones and the significant impact on mobility and quality of life. Tendinopathy-related symptoms often require consistent clinical monitoring, rehabilitation programs, and supportive care, which drives high demand in hospitals and specialty centers. Awareness campaigns and updated clinical guidelines emphasizing early detection and management further reinforce this segment’s dominance. Healthcare providers prioritize treatments that can effectively mitigate tendon damage and accelerate recovery, ensuring steady adoption. Patient education on avoiding overexertion and using supportive therapies also contributes to sustained growth. In addition, pharmaceutical innovations targeting tendon protection and recovery are strengthening market positioning.
The Peripheral Neuropathy segment is anticipated to witness the fastest CAGR of 21.8% from 2025 to 2032, driven by increasing recognition of neuropathic complications from fluoroquinolone exposure. Peripheral neuropathy can cause persistent pain, numbness, and motor impairments, prompting higher demand for early diagnosis and therapeutic interventions. Rising awareness among clinicians and patients, coupled with ongoing research into neuroprotective treatments, is accelerating adoption. Homecare programs and outpatient monitoring solutions facilitate better management of neuropathy symptoms. The growing prevalence of chronic conditions that predispose patients to neuropathic side effects is also fueling market expansion. Educational initiatives and integration with telehealth solutions are further boosting the segment’s growth trajectory.
- By Diagnosis Method
On the basis of diagnosis method, the fluoroquinolone toxicity syndrome market is segmented into clinical examination, laboratory testing, and imaging techniques. The clinical examination segment accounted for the largest market revenue share of 46.5% in 2024, driven by its wide adoption in hospitals and clinics for early detection of toxicity symptoms. Clinical examination allows healthcare providers to identify musculoskeletal, neurological, and cardiovascular manifestations without reliance on advanced equipment, making it accessible across a broad range of healthcare settings. The structured use of physical assessments, patient history evaluation, and symptom monitoring ensures accurate diagnosis. Hospitals and specialty centers benefit from standardized clinical protocols that enhance consistency and patient outcomes. Regular training of healthcare personnel on symptom recognition further strengthens adoption. Integration with electronic health records and guideline-based care also improves efficiency.
The Laboratory Testing segment is expected to witness the fastest CAGR of 20.9% from 2025 to 2032, due to increasing demand for precise quantification of biomarkers and biochemical indicators of toxicity. Laboratory tests enable early detection of systemic effects, support differential diagnosis, and guide targeted interventions. Rising availability of automated and high-throughput testing platforms enhances workflow efficiency. Homecare and specialty centers are also increasingly incorporating lab-based monitoring to improve patient safety. Awareness programs and clinical research demonstrating laboratory testing efficacy are contributing to rapid adoption. Additionally, insurance coverage and cost-effective lab solutions make this segment more accessible.
- By Treatment Approach
On the basis of treatment approach, the Fluoroquinolone toxicity syndrome market is segmented into symptomatic treatment, physical therapy, and surgical intervention. The Symptomatic Treatment segment dominated the largest market revenue share of 44.8% in 2024, attributed to the broad applicability of medications and supportive care to manage pain, inflammation, and gastrointestinal effects. Symptomatic treatment is commonly adopted across hospitals, clinics, and homecare, providing a flexible approach tailored to patient needs. Guidelines recommend prompt intervention with anti-inflammatory agents, analgesics, and adjunct therapies to minimize complications. Widespread availability, cost-effectiveness, and ease of administration contribute to sustained market dominance. Patient adherence programs and continuous updates in clinical protocols further reinforce the segment’s growth. Health professionals prefer symptom-focused interventions for rapid relief and to prevent progression of more severe toxicity.
The Physical Therapy segment is anticipated to witness the fastest CAGR of 22.0% from 2025 to 2032, driven by increasing incidence of musculoskeletal complications and tendon injuries. Physical therapy aids in restoring mobility, improving muscle strength, and preventing long-term disability. Growing recognition of rehabilitation as an essential component of toxicity management is fueling adoption. Hospitals, specialty centers, and homecare providers are expanding therapeutic programs. Technological advancements in physiotherapy devices and virtual rehabilitation solutions are accelerating growth. Patient education, insurance support, and integration with comprehensive care plans further enhance the uptake of physical therapy interventions.
- By Risk Factors
On the basis of risk factors, the fluoroquinolone toxicity syndrome market is segmented into age, gender, and pre-existing conditions. The Age segment dominated the largest market revenue share of 47.0% in 2024, driven by higher susceptibility among older patients to adverse effects of fluoroquinolones. Elderly populations often experience impaired drug metabolism, leading to increased risk of tendinopathy, neuropathy, and cardiac issues. Hospitals and clinics prioritize monitoring for high-risk age groups, reinforcing segment dominance. Awareness campaigns, geriatric-specific treatment protocols, and age-focused clinical research contribute to continued adoption. The integration of risk assessment in patient management systems ensures proactive intervention. Pharmaceutical innovations targeting age-specific toxicity mitigation further support this segment.
The Pre-existing Conditions segment is expected to witness the fastest CAGR of 21.5% from 2025 to 2032, due to rising prevalence of comorbidities such as diabetes, cardiovascular disease, and renal dysfunction. Patients with pre-existing conditions have an elevated risk of severe toxicity, necessitating careful monitoring and specialized treatment approaches. Hospitals, homecare providers, and specialty centers increasingly adopt tailored management plans. Awareness programs highlighting risk factor identification and early intervention further drive adoption. The growing number of complex cases and integration with diagnostic testing enhance the segment’s growth prospects.
- By End-Users
On the basis of end-users, the fluoroquinolone toxicity syndrome market is segmented into hospitals, homecare, specialty centres, and others. The Hospitals segment accounted for the largest market revenue share of 48.2% in 2024, due to the high volume of patients requiring controlled, monitored care and rapid intervention. Hospitals prefer structured treatment regimens and pharmacovigilance programs, ensuring consistent utilization. Bulk procurement, clinical oversight, and adherence to treatment protocols reinforce their dominant position. Hospitals’ capacity to administer combination therapies under supervision improves outcomes and patient safety. Continuous medical education, updated clinical guidelines, and protocol standardization further enhance adoption. Integration of monitoring technologies and inpatient care ensures timely management of toxicity symptoms.
The Homecare segment is anticipated to witness the fastest CAGR of 20.8% from 2025 to 2032, driven by the rising preference for outpatient management and patient-managed care. Homecare allows patients to manage mild-to-moderate symptoms at home, reducing hospital visits and overall healthcare costs. Increasing telehealth consultations, remote monitoring solutions, and access to medications facilitate this growth. Patient and caregiver education, adherence support programs, and the convenience of at-home care accelerate adoption. The integration of homecare with clinical oversight ensures safety while expanding access. Awareness campaigns emphasizing home-based management benefits further drive market growth.
- By Distribution Channel
On the basis of distribution channel, the fluoroquinolone toxicity syndrome market is segmented into hospital pharmacy, online pharmacy, retail pharmacy, and others. The Hospital Pharmacy segment dominated the largest market revenue share of 47.5% in 2024, driven by direct procurement for inpatient care, monitored therapeutic use, and adherence to clinical protocols. Hospitals ensure prompt administration, bulk availability, and controlled access. Strong relationships with pharmaceutical manufacturers and distributors reinforce segment dominance. Standardized formulations like Kaolin maintain high demand. Hospital formularies, treatment guideline updates, and clinical oversight further strengthen market share.
The Online Pharmacy segment is expected to witness the fastest CAGR of 21.7% from 2025 to 2032, fueled by increasing adoption of e-commerce, home delivery of medications, and accessibility for remote patients. Online platforms offer convenience, dosage guidance, adherence reminders, and integration with telehealth services. Growing digital literacy, secure payment systems, and awareness campaigns encourage patient adoption. Access to essential therapies for mild-to-moderate cases outside hospitals further accelerates growth. Cost-effectiveness, convenience, and wider geographic reach collectively contribute to the rapid expansion of this segment.
Fluoroquinolone Toxicity Syndrome Market Regional Analysis
- North America dominated the fluoroquinolone toxicity syndrome market with the largest revenue share of 40.5% in 2024, supported by advanced healthcare infrastructure, widespread adoption of diagnostic and monitoring services, and the strong presence of pharmaceutical companies investing in safer therapeutic alternatives
- The increasing prevalence of fluoroquinolone prescriptions for infectious diseases and the need to manage associated toxicity effectively is further fueling market growth
- Hospitals and specialty clinics are leading the adoption of safer treatment protocols, while continuous medical education ensures healthcare professionals are aware of adverse drug reactions. The region’s well-established regulatory framework and reimbursement policies for monitoring adverse drug effects also encourage market expansion
U.S. Fluoroquinolone Toxicity Syndrome Market Insight
The U.S. fluoroquinolone toxicity syndrome market captured the largest revenue share within North America, with 85% in 2024, driven by widespread uptake of pharmacovigilance programs, molecular diagnostics, and advanced cytogenetic techniques. Awareness campaigns and clinical guidelines promoting early detection of fluoroquinolone toxicity are accelerating adoption across hospitals and specialty centers. The demand for safer therapeutic alternatives and monitoring solutions continues to grow, with major pharmaceutical companies actively investing in research and patient education. Increasing patient awareness about adverse effects and the integration of hospital-based monitoring programs further support market expansion. In addition, the presence of well-equipped hospitals, trained healthcare professionals, and comprehensive healthcare coverage contributes to the strong market performance in the U.S.
Europe Fluoroquinolone Toxicity Syndrome Market Insight
The Europe fluoroquinolone toxicity syndrome market is projected to expand at a substantial CAGR during the forecast period, driven by the region’s stringent regulatory environment and the emphasis on patient safety in antibiotic prescriptions. Growing urbanization, rising awareness among healthcare providers, and increased adoption of monitoring services are supporting market growth. Hospitals, diagnostic centers, and specialty clinics across the UK, Germany, France, and other European countries are increasingly adopting guidelines for the prevention and management of fluoroquinolone-associated toxicity. Enhanced awareness programs and educational initiatives aimed at minimizing adverse drug reactions are further fostering adoption. The market is experiencing growth in both inpatient and outpatient care settings, reflecting a comprehensive approach to managing fluoroquinolone toxicity.
U.K. Fluoroquinolone Toxicity Syndrome Market Insight
The U.K. fluoroquinolone toxicity syndrome market is expected to grow at a noteworthy CAGR throughout the forecast period, supported by proactive patient safety initiatives and robust healthcare infrastructure. Increasing awareness of potential adverse effects from fluoroquinolone antibiotics is driving physicians to adopt safer monitoring practices. Hospitals and specialty centers are prioritizing pharmacovigilance programs and early intervention strategies to prevent severe toxicity. In addition, well-established guidelines and growing patient education programs are encouraging adoption across healthcare facilities. The increasing use of clinical diagnostics for early detection of fluoroquinolone toxicity also contributes to market growth.
Germany Fluoroquinolone Toxicity Syndrome Market Insight
The Germany fluoroquinolone toxicity syndrome market is anticipated to expand at a considerable CAGR, fueled by advanced healthcare services, strict regulatory frameworks, and rising awareness of adverse drug reactions. Hospitals, clinics, and diagnostic centers are increasingly implementing monitoring programs to detect and manage fluoroquinolone toxicity. There is strong adoption of clinical guidelines and therapeutic alternatives to mitigate risks associated with fluoroquinolone use. The emphasis on patient safety and proactive pharmacovigilance contributes to steady market growth. Growing adoption in outpatient settings and integration with healthcare IT systems for monitoring adverse effects are also driving expansion. Germany’s well-developed healthcare infrastructure supports effective management of drug-related toxicity.
Asia-Pacific Fluoroquinolone Toxicity Syndrome Market Insight
The Asia-Pacific fluoroquinolone toxicity syndrome market is poised to grow at the fastest CAGR of 9.1% from 2025 to 2032, driven by increasing urbanization, rising disposable incomes, expansion of healthcare facilities offering specialized infectious disease management, and growing awareness among patients and physicians regarding adverse drug reactions related to fluoroquinolones. Countries such as China, Japan, and India are experiencing a surge in the adoption of diagnostic and monitoring services to manage fluoroquinolone-associated toxicity. Governments and healthcare institutions are actively promoting awareness programs and training for healthcare professionals. Rapid development of hospital and specialty care infrastructure, combined with better access to pharmaceuticals, is supporting regional growth. Moreover, increasing patient education and focus on safer therapeutic practices are further driving market expansion.
Japan Fluoroquinolone Toxicity Syndrome Market Insight
The Japan fluoroquinolone toxicity syndrome market is witnessing steady growth due to a high prevalence of antibiotic prescriptions, strong healthcare infrastructure, and growing awareness of fluoroquinolone toxicity among healthcare professionals. Hospitals and clinics are implementing monitoring and early intervention programs to manage adverse reactions effectively. The aging population, coupled with increasing antibiotic use, is driving demand for safer therapeutic alternatives. National healthcare policies emphasizing patient safety and pharmacovigilance are further supporting market growth. Continuous training programs for healthcare providers ensure the adoption of effective monitoring and treatment protocols. Japan’s focus on patient-centric care and clinical research contributes to the steady expansion of the market.
China Fluoroquinolone Toxicity Syndrome Market Insight
The China fluoroquinolone toxicity syndrome market is expected to witness the highest CAGR in the Asia-Pacific region during the forecast period, driven by expanding healthcare infrastructure, rapid urbanization, increasing demand for specialized infectious disease management, and a growing middle-class population. Hospitals and specialty centers are increasingly adopting protocols for early detection and management of fluoroquinolone toxicity. Government initiatives promoting patient safety, along with increased access to diagnostic services, are further fueling growth. Awareness campaigns targeting both healthcare providers and patients are enhancing adoption rates. In addition, ongoing investments in advanced healthcare facilities and regional expansion of pharmaceutical companies strengthen market opportunities. Affordability and accessibility of monitoring services are enabling wider patient coverage, accelerating the market’s growth trajectory.
Fluoroquinolone Toxicity Syndrome Market Share
The fluoroquinolone toxicity syndrome industry is primarily led by well-established companies, including:
- Bayer AG (Germany)
- Johnson & Johnson and its affiliates (U.S.)
- Merck & Co., Inc. (U.S.)
- Pfizer Inc. (U.S.)
- F. Hoffmann-La Roche AG (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Viatris Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- GSK plc (U.K.)
- AstraZeneca plc (U.K.)
- Lilly USA, LLC (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- AbbVie Inc. (U.S.)
- Novartis AG (Switzerland)
- Sanofi S.A. (France)
Latest Developments in Global Fluoroquinolone Toxicity Syndrome Market
- In March 2025, the Australian Therapeutic Goods Administration (TGA) issued a safety update highlighting that fluoroquinolones, including ciprofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions involving different body systems. These reactions have occurred together in the same patient and have been reported in patients of any age or without pre-existing risk factors. The TGA emphasized the need for healthcare professionals to be aware of these risks when prescribing fluoroquinolones
- In October 2025, the Centers for Disease Control and Prevention (CDC) approved new ICD-10 medical billing codes for fluoroquinolone toxicity, effective from October 2025. This development is expected to improve the documentation and reporting of adverse effects related to fluoroquinolones, aiding in better patient management and research
- In January 2024, the UK Medicines and Healthcare products Regulatory Agency (MHRA) introduced new restrictions for systemic fluoroquinolones. This was part of the agency's third drug safety update on these antibiotics in the past six months, responding to an increase in reports of suspected musculoskeletal side effects associated with ciprofloxacin
- In February 2024, the European Medicines Agency (EMA) issued updated guidelines on the use of fluoroquinolones, recommending stricter restrictions on their use due to concerns over serious side effects. The guidelines aim to balance the benefits of these antibiotics with the risks associated with their use
- In January 2023, the UK Medicines and Healthcare products Regulatory Agency (MHRA) initiated a review of the use of fluoroquinolones, engaging with patient advocacy groups such as Fluoroquinolone Toxicity Support UK (FQTSUK). This review aimed to gather patient experiences and assess the safety profile of these antibiotics, reflecting a growing emphasis on patient-centered approaches in regulatory decision-making
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.