Global Gastroenteritis Testing Market
Market Size in USD Billion
CAGR :
% 
 USD
4.18 Billion
USD
6.18 Billion
2024
2032
USD
4.18 Billion
USD
6.18 Billion
2024
2032
| 2025 –2032 | |
| USD 4.18 Billion | |
| USD 6.18 Billion | |
|
|
|
|
Gastroenteritis Testing Market Size
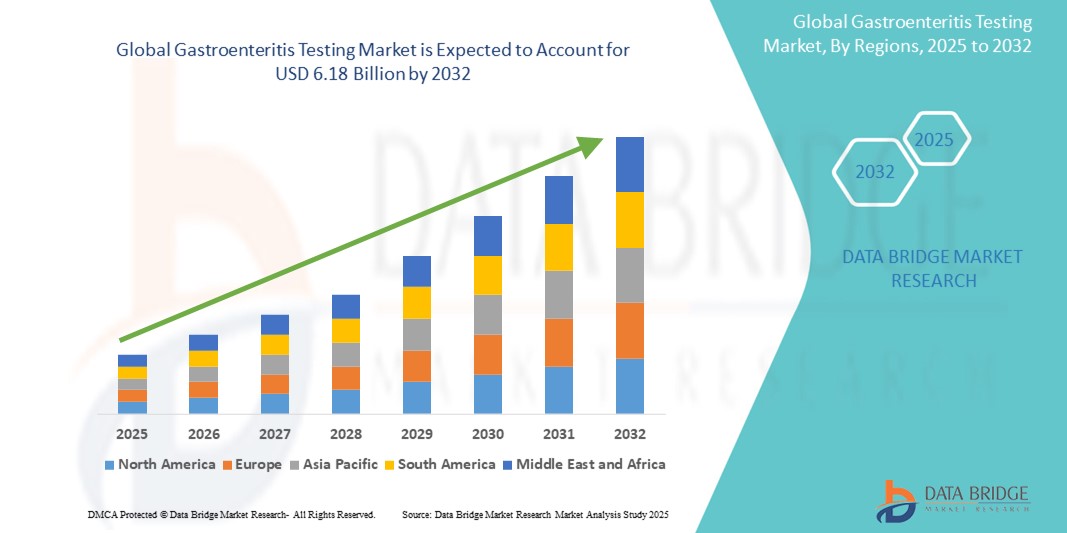
- The global gastroenteritis testing market size was valued at USD 4.18 billion in 2024 and is expected to reach USD 6.18 billion by 2032, at a CAGR of 5.00% during the forecast period
- The market growth is primarily driven by the increasing global burden of gastrointestinal infections, rising awareness about early diagnosis, and the widespread implementation of diagnostic screening programs in both developed and developing regions
- Moreover, the demand for rapid, accurate, and multiplex testing solutions for pathogens such as norovirus, rotavirus, and bacterial agents is pushing technological advancements and adoption rates. These dynamics are reinforcing the critical role of gastroenteritis testing in public health surveillance and clinical diagnosis, thereby accelerating overall market expansion
Gastroenteritis Testing Market Analysis
- Gastroenteritis testing, which involves the detection of viral, bacterial, or parasitic pathogens responsible for gastrointestinal infections, is becoming an essential part of public health management and clinical diagnostics due to its ability to enable early and accurate treatment decisions
- The growing demand for gastroenteritis testing is primarily driven by the increasing prevalence of gastrointestinal diseases, rising global food and water contamination incidents, and heightened awareness regarding timely and accurate diagnostics
- North America dominated the gastroenteritis testing market with the largest revenue share of 40% in 2024, supported by well-established healthcare infrastructure, advanced diagnostic technologies, and widespread access to clinical laboratories, with the U.S. leading in test volumes owing to routine screening protocols and investments in molecular diagnostics
- Asia-Pacific is expected to be the fastest growing region in the gastroenteritis testing market during the forecast period due to expanding healthcare access, increasing government initiatives for infectious disease control, and rising healthcare expenditure
- The molecular diagnostics segment dominated the gastroenteritis testing market with a market share of 42% in 2024, attributed to its superior accuracy, speed, and ability to simultaneously detect multiple pathogens, making it the preferred choice in hospital and reference lab settings
Report Scope and Gastroenteritis Testing Market Segmentation
|
Attributes |
Gastroenteritis Testing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Gastroenteritis Testing Market Trends
“Shift Toward Multiplex and Point-of-Care Diagnostics”
- A significant and accelerating trend in the global gastroenteritis testing market is the growing adoption of multiplex molecular diagnostics and rapid point-of-care (POC) testing technologies, which offer faster and more comprehensive detection of gastrointestinal pathogens
- For instance, the BioFire FilmArray Gastrointestinal Panel allows clinicians to detect more than 20 different viral, bacterial, and parasitic pathogens in a single test within approximately one hour, enabling quicker and more accurate treatment decisions. Similarly, Quidel’s Sofia® platform delivers rapid results through easy-to-use antigen testing formats, particularly suited for outpatient and urgent care settings
- Multiplex diagnostic tools enhance clinical efficiency by reducing the need for multiple, time-consuming tests, while improving accuracy and guiding appropriate antimicrobial usage. These solutions are particularly valuable during outbreaks, where quick pathogen identification is crucial for controlling transmission
- Furthermore, the demand for point-of-care testing is rising in decentralized healthcare environments, including rural clinics and low-resource regions, due to their portability and minimal requirement for laboratory infrastructure
- The integration of user-friendly diagnostic platforms with high sensitivity and specificity is reshaping diagnostic workflows. Companies such as Cepheid and Meridian Bioscience are actively expanding their test menus and investing in innovations that combine speed, automation, and pathogen breadth
- This growing emphasis on multiplex and rapid diagnostics is transforming the gastroenteritis testing landscape by delivering timely results, reducing clinical burden, and improving outcomes, particularly in high-risk populations and resource-limited settings
Gastroenteritis Testing Market Dynamics
Driver
“Rising Prevalence of GI Infections and Public Health Initiatives”
- The increasing global incidence of gastrointestinal infections and the expansion of public health screening programs are major drivers for the gastroenteritis testing market’s growth
- For instance, initiatives by the World Health Organization (WHO) and regional health agencies to combat foodborne and waterborne diseases are prompting enhanced diagnostic capabilities in hospitals and laboratories
- The widespread occurrence of pathogens such as rotavirus, norovirus, Clostridium difficile, and Campylobacter, especially among vulnerable populations such as children and the elderly, is intensifying the demand for early and reliable diagnostic testing
- Hospitals and clinics are implementing more robust screening protocols to quickly identify causative agents, manage infections effectively, and reduce transmission in community and healthcare settings
- Moreover, the increased availability of rapid and multiplex diagnostic platforms is improving detection rates and enabling clinicians to make timely decisions, thereby reducing unnecessary antibiotic use and promoting targeted therapies
- Government support, growing awareness about hygiene and sanitation, and rising healthcare investments especially in emerging economies are further supporting the widespread implementation of gastroenteritis testing
Restraint/Challenge
“Limited Access and High Cost in Low-Resource Settings”
- Despite technological advances, the high cost of advanced diagnostic platforms and limited infrastructure in low-income and rural areas remain key challenges to the broad adoption of gastroenteritis testing
- For instance, molecular diagnostic systems require specialized equipment and trained personnel, which are often lacking in underdeveloped regions. This creates delays in diagnosis and contributes to underreporting of infection rates
- In addition, the cost of high-end multiplex panels and automated analyzers can be prohibitive for smaller clinics and public health systems operating under budget constraints
- Many healthcare providers in resource-limited areas still rely on symptom-based diagnosis or basic stool microscopy, which lack the accuracy and speed needed for effective treatment
- The absence of standardized diagnostic protocols and inconsistent access to quality testing materials further compound the issue, limiting the ability to track and respond to outbreaks efficiently
- Addressing these barriers will require investment in affordable diagnostic technologies, expansion of decentralized testing networks, public-private partnerships, and capacity-building initiatives to improve access and affordability in underserved regions
Gastroenteritis Testing Market Scope
The market is segmented on the basis of disease strains, product type, testing methods, and end-user.
- By Disease Strains
On the basis of disease strains, the gastroenteritis testing market is segmented into bacterial strains, viral strains, and parasitic strains. The bacterial strains segment dominated the market with the largest revenue share in 2024, attributed to the high global prevalence of bacterial gastroenteritis caused by pathogens such as Escherichia coli, Salmonella, Shigella, and Campylobacter. These infections often require specific treatment protocols and can result in severe outbreaks, particularly in developing regions with inadequate sanitation.
The viral strains segment is expected to witness the fastest growth rate from 2025 to 2032, driven by rising incidences of norovirus and rotavirus infections, especially in pediatric and geriatric populations. Increasing awareness and the introduction of rapid, multiplex tests for viral detection are also fueling growth in this segment.
- By Product Type
On the basis of product type, the gastroenteritis testing market is segmented into reagent kits, sequencing kits, and equipment. The reagent kits segment held the largest market revenue share in 2024, due to their wide usage in routine diagnostics and compatibility with various testing platforms including immunoassays and PCR-based diagnostics. Their ease of use, affordability, and availability for targeted detection of specific pathogens make them the most preferred option in clinical laboratories.
The sequencing kits segment is projected to grow at the fastest CAGR during the forecast period, owing to rising adoption of next-generation sequencing (NGS) in pathogen identification and microbiome analysis. This growth is supported by the increasing emphasis on genomic surveillance and outbreak investigation, particularly for identifying antibiotic resistance genes and emerging pathogens.
- By Testing Methods
On the basis of testing methods, the gastroenteritis testing market is segmented into immunoassay testing, conventional testing, and molecular diagnostic testing. The molecular diagnostic testing segment dominated the market with the highest share of 42% in 2024, as it offers superior accuracy, sensitivity, and the capability to detect multiple pathogens simultaneously through multiplex assays. The demand for these tests is rising in hospitals and high-throughput diagnostic labs due to their rapid turnaround and comprehensive results.
The immunoassay testing segment is projected to grow at the fastest CAGR during the forecast period, driven by its affordability and application in point-of-care testing environments. Lateral flow immunoassays and ELISA-based kits are widely used for their quick results and minimal equipment requirements, especially in resource-limited settings.
- By End User
On the basis of end-user, the gastroenteritis testing market is segmented into hospitals, clinics/medical centers, research institutes, and diagnostic laboratories. The hospitals segment accounted for the largest market share in 2024 owing to the high volume of patient admissions, the need for immediate diagnosis, and the availability of in-house laboratories equipped with advanced testing capabilities. Hospitals play a crucial role in managing acute gastroenteritis outbreaks and ensuring prompt clinical decision-making.
The diagnostic laboratories segment is expected to register the fastest growth from 2025 to 2032, supported by increasing test volumes, outsourcing trends in healthcare diagnostics, and the availability of automated, high-throughput testing systems. Central labs are also adopting multiplex panels and molecular platforms for large-scale screening and public health surveillance.
Gastroenteritis Testing Market Regional Analysis
- North America dominated the gastroenteritis testing market with the largest revenue share of 40% in 2024, supported by well-established healthcare infrastructure, advanced diagnostic technologies, and widespread access to clinical laboratories
- Consumers and healthcare providers in the region prioritize rapid, reliable diagnostic tools, with widespread adoption of molecular and multiplex testing platforms in hospitals, diagnostic labs, and outpatient settings
- This strong market position is further reinforced by favorable reimbursement policies, growing awareness of infectious disease control, and continuous innovation by regional diagnostic companies, making North America a key hub for advanced gastroenteritis testing solutions in both clinical and public health applications
U.S. Gastroenteritis Testing Market Insight
The U.S. gastroenteritis testing market captured the largest revenue share of 79% in 2024 within North America, driven by the high incidence of gastrointestinal infections, widespread implementation of routine diagnostic testing, and advanced molecular diagnostic capabilities. The demand for rapid and accurate pathogen detection tools is growing, particularly in hospitals, urgent care centers, and public health labs. Continued investment in healthcare infrastructure and R&D, along with proactive government surveillance programs, further supports market growth. The presence of leading diagnostic firms and their focus on multiplex and point-of-care testing solutions significantly contribute to the U.S. market’s expansion.
Europe Gastroenteritis Testing Market Insight
The Europe gastroenteritis testing market is projected to expand at a substantial CAGR throughout the forecast period, fueled by stringent infectious disease regulations and an increased emphasis on early and precise diagnosis. Rising foodborne illness cases and hospital-acquired infections are prompting the adoption of advanced diagnostic tools. The region’s focus on universal healthcare access and public health initiatives is boosting the demand for comprehensive testing solutions across hospitals and laboratories. Multiplex PCR and automated systems are gaining traction across both public and private healthcare facilities.
U.K. Gastroenteritis Testing Market Insight
The U.K. gastroenteritis testing market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by the country’s commitment to infection control and widespread adoption of modern diagnostic technologies. National surveillance programs and NHS investments in pathogen detection platforms are key growth drivers. The rising prevalence of norovirus outbreaks, especially in schools and care homes, is increasing demand for rapid diagnostics. Furthermore, the U.K.'s centralized healthcare system ensures consistent testing protocols, supporting efficient outbreak tracking and treatment.
Germany Gastroenteritis Testing Market Insight
The Germany gastroenteritis testing market is expected to expand at a considerable CAGR during the forecast period, driven by a strong healthcare infrastructure, high diagnostic awareness, and growing demand for molecular testing solutions. Germany’s focus on public health, food safety, and hygiene practices fosters the widespread use of diagnostic tools across hospitals, research institutes, and public labs. The country is also investing in innovations such as real-time PCR and sequencing for precise pathogen identification, which is increasing the adoption of high-end testing solutions.
Asia-Pacific Gastroenteritis Testing Market Insight
The Asia-Pacific gastroenteritis testing market is poised to grow at the fastest CAGR of 23.5% during the forecast period of 2025 to 2032, driven by rising gastrointestinal disease prevalence, improved healthcare access, and technological advancements in countries such as China, Japan, and India. Government-led healthcare modernization and disease surveillance initiatives are expanding diagnostic capacities. Increasing awareness about food and waterborne infections, coupled with rising healthcare expenditure and urbanization, is driving the adoption of accurate and rapid testing platforms across the region.
Japan Gastroenteritis Testing Market Insight
The Japan gastroenteritis testing market is gaining momentum due to its advanced healthcare system, aging population, and increasing use of automated and multiplex diagnostic platforms. The country’s strong focus on infection prevention and early intervention encourages widespread testing, particularly in hospitals and elderly care facilities. High demand for compact, efficient diagnostic tools compatible with Japan’s tech-driven healthcare infrastructure is supporting market expansion. Public health campaigns targeting hygiene and food safety are also boosting awareness and testing rates.
India Gastroenteritis Testing Market Insight
The India gastroenteritis testing market accounted for the largest market revenue share in Asia-Pacific in 2024, driven by the country’s large population, improving healthcare infrastructure, and rising awareness of infectious disease diagnostics. The government’s initiatives to combat diarrheal diseases and enhance primary care services have accelerated the deployment of diagnostic tools in public health centers. Affordable test kits and rising penetration of point-of-care solutions are enabling broader access across urban and rural areas. In addition, the local manufacturing of testing kits and the growth of private diagnostics chains are further propelling market expansion in India.
Gastroenteritis Testing Market Share
The gastroenteritis testing industry is primarily led by well-established companies, including:
- BD (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sanofi (France)
- Pfizer Inc. (U.S.)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- AstraZeneca (U.K.)
- Johnson & Johnson Services, Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Merck & Co., Inc. (U.S.)
- Lilly (U.S.)
- Amgen Inc. (U.S.)
- Hainan Poly Co. Ltd (China)
- WOCKHARDT (India)
What are the Recent Developments in Global Gastroenteritis Testing Market?
- In April 2023, BioMérieux, a global leader in in-vitro diagnostics, launched its advanced VIDAS Gastrointestinal Panel, a rapid immunoassay solution for the detection of major gastroenteritis-causing pathogens. The panel is designed to deliver quick and accurate results for both bacterial and viral strains, enhancing diagnostic efficiency in clinical settings. This innovation strengthens BioMérieux’s position in the molecular diagnostics space and aligns with the growing demand for multiplex testing in gastrointestinal disease management
- In March 2023, QuidelOrtho Corporation announced the expansion of its Sofia 2 Analyzer platform with a new test for norovirus detection. The launch is intended to meet the increasing need for rapid diagnostics in outpatient clinics and emergency departments, particularly during seasonal outbreaks. With a turnaround time of under 15 minutes, this addition underscores QuidelOrtho’s commitment to developing reliable, point-of-care solutions for acute gastroenteritis cases
- In March 2023, Thermo Fisher Scientific Inc. unveiled its latest TaqPath Enteric Pathogen Panels, offering real-time PCR-based multiplex testing for gastrointestinal pathogens. Designed for high-throughput laboratories, the panels cover a wide array of viral, bacterial, and parasitic targets. This launch addresses growing concerns over antimicrobial resistance by supporting more precise pathogen identification, further reinforcing Thermo Fisher’s leadership in molecular diagnostics innovation
- In February 2023, Luminex Corporation announced a partnership with a consortium of public health labs in Southeast Asia to deploy its xTAG Gastrointestinal Pathogen Panel (GPP) for enhanced disease surveillance. The initiative is aimed at improving diagnostic capabilities in regions with high gastroenteritis prevalence, and emphasizes Luminex’s role in advancing molecular diagnostic access in underserved markets
- In January 2023, Cepheid, a subsidiary of Danaher Corporation, received regulatory clearance for its Xpert Xpress Gastro Panel in the European market. The panel enables rapid, cartridge-based detection of multiple enteric pathogens using minimal hands-on time. Designed to support decentralized testing environments such as urgent care centers and remote hospitals, this development highlights Cepheid’s focus on combining speed, accuracy, and accessibility to improve clinical outcomes in gastroenteritis management
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.