Global Generic Drug Market
Market Size in USD Million
CAGR :
% 
 USD
622.02 Million
USD
1,323.68 Million
2022
2030
USD
622.02 Million
USD
1,323.68 Million
2022
2030
| 2023 –2030 | |
| USD 622.02 Million | |
| USD 1,323.68 Million | |
|
|
|
|
Generic Drug Market Analysis and Size
The World Health Organization estimates that each year there are between 2 and 3 million instances of non-melanoma skin cancer and 132,000 cases of melanoma skin cancer. In addition, the prevalence of psoriasis in the world varies from 0.09 percent to 11.43%, making it a serious condition that affects at least 100 million people worldwide. The fact is that topical drug administration is the primary therapy method for most skin conditions, and the market for advanced topical products is projected to grow in the upcoming years.
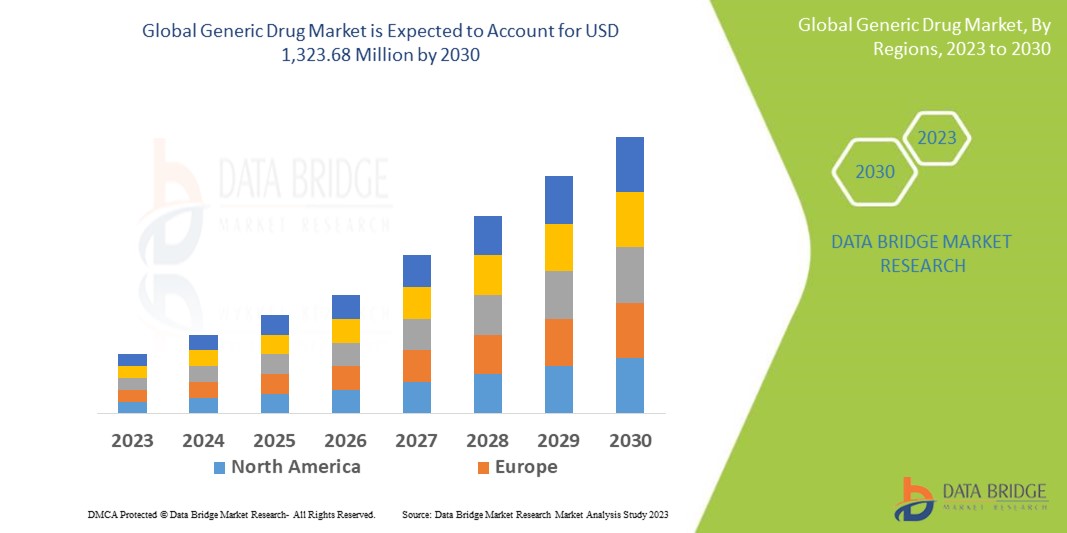
Data Bridge Market Research analyses that the generic drug market, which is USD 622.02 million in 2022, is expected to reach USD 1,323.68 million by 2030, at a CAGR of 9.9% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Generic Drug Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Simple Generics, Super Generics), Brand (Pure Generic, Branded Generic), Indication (Central Nervous System (CNS), Cardiovascular, Dermatology, Oncology, Respiratory Others), Route of Administration (Oral, Topical, Parenteral, Others), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Teva Pharmaceuticals Industries Ltd., (Israel), Mylan NV, (US), Novartis AG, (Switzerland), Pfizer Inc., (US), Sun Pharmaceutical Industries Ltd., (India), Fresenius SE & Co. KGaA., (Germany), Lupin (India), Endo International plc., (Ireland), Aurobindo Pharma (India), Novartis AG (Switzerland), Hikma Pharmaceuticals PLC., (UK), STADA Arzneimittel AG (Germany), Eli Lilly and Company (US) and Aspen Holdings (South Africa) |
|
Market Opportunities |
|
Market Definition
Generic medications differ from branded drugs in several aspects, such as the manufacturing process utilized in drug development, excipients, and packaging. Still, they are bioequivalent to branded drugs in terms of strength, dose, quality, safety, performance, and efficacy. Generic drugs become available when the patents for already marketed medications expire. Generic medications, not associated with a particular manufacturer, are often controlled by governments worldwide.
Generic Drug Market Dynamics
Drivers
- Rise in demand for artificial intelligence (AI) technology will bolster the mmarket growth
Robotic process automation uses artificial intelligence (AI) technology to automate routine, rules-based activities. The market's primary operational players can invest more time, effort, and resources in higher-value tasks to this automation. One of the key developments in the market for generic brands that will gain pace in the upcoming years is the use of RPA to ensure compliance with regulations and standards. Pharmaceutical companies frequently utilize RPA and other business process automation tools to carry out high-volume R&D and production tasks. These are the certain factors that propel the market growth.
- India’s thriving pharmaceutical industry propelling India generic drugs market
One of the biggest pharmaceutical markets in the world, India is home to several top pharmaceutical firms. The Indian pharmaceutical sector is ranked third globally in terms of volume and fourteenth globally in terms of value, according to Invest India, an agency of the Indian Government for Investment Promotion and Facilitation. Along with having more than 3,000 pharmaceutical businesses and more than 10,500 production facilities, India also has the most US-FDA compliant pharmaceutical plants outside of the United States.
Opportunities
- Rise in cancer cases will act as an opportunity
According to the International Agency for Research on Cancer's (IARC) 2020 report, which estimated the incidence and mortality of 36 cancers in 185 countries worldwide, an estimated 19.3 million new cases of cancer were diagnosed in 2020 throughout the world, with more than 10.1 million cases of cancer reported in men and 9.3 million cases in women. Additionally, the Global RA Network's 2021 study estimates that more than 350 million individuals worldwide have arthritis. That number is likely to rise due to several variables, including the growing global elderly population. As a result, it is anticipated that the rising prevalence of chronic diseases will increase the need for effective treatment, driving up the growth of the generic drug market throughout the forecast period.
Restraints/Challenges
- Stringent regulations will hinders the growth
Stringent controls, as the FDA assesses the accuracy, side effects, and other substances used in generic pharmaceuticals, are one of the main factors limiting the expansion of generic drugs. Pharmaceutical drugs are typically recalled if the producers don't follow the regulatory requirements. The key elements that influence the quality of generic drugs are purity, potency, stability, and drug release. These should be managed within an appropriate limit, range, or distribution to obtain the desired drug quality. As a result of the strict governmental rules, approval is needed for generic pharmaceuticals, which is projected to hinder market expansion.
This generic drug market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the generic drug market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on the Generic Drug Market
The COVID-19 epidemic had a significant influence on the generic drug market. Following the COVID-19 pandemic, practically every business initially encountered supply chain interruptions due to various social distancing regulations. The pharmaceutical industry was no exception, which had a detrimental impact on the generic medication market. Later, due to the COVID-19 illness's multiple opportunities to generate drugs to treat this infection, the market witnessed an increase in demand for generic pharmaceuticals. The US Food and Drug Administration (FDA) established the Generic Drug Program to help generic drug producers develop new products by sending written messages, attending meetings early in the development process, and clarifying regulatory requirements throughout application review.
Recent Developments
- In 2020, ANI Pharmaceuticals, Inc., a US-based integrated specialty pharmaceutical company focused on developing, producing, and marketing high-quality branded and generic prescription pharmaceuticals acquired Commercial and Pipeline Generic Products from Amerigen Pharmaceuticals, Ltd. for $52.5 million in cash. The deal has greatly increased ANI Pharmaceuticals, Inc's commercial portfolio and late-stage generic pipeline.
Global Generic Drug Market Scope
The generic drug market is segmented on the basis of type, brand, indication, route of administration, end-users and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Simple Generics
- Super Generics
Brand
- Pure Generic
- Branded Generic
Indication
- Central Nervous System (CNS)
- Cardiovascular
- Dermatology
- Oncology
- Respiratory
- Others
Route of Administration
- Oral
- Topical
- Parenteral
- Others
End-Users
- Hospitals
- Homecare
- Specialty Clinics
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Generic Drug Market Regional Analysis/Insights
The generic drug market is analysed and market size insights and trends are provided by country, type, brand, indication, route of administration, end-users and distribution channel as referenced above.
The countries covered in the generic drug market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the generic drug market due to the region's established framework for the approval process of generic medications and global leaders in research and development activities.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030, owing to the expanding healthcare infrastructure and rise in the government initiatives. Also due to rise in medical condition knowledge among the general public and an ageing regional population. In the Asia-Pacific area, countries such as China and India contribute more than other countries do.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Generic Drug Market Share Analysis
The generic drug market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to generic drug market.
Some of the major players operating in the generic drug market are:
- Teva Pharmaceuticals Industries Ltd., (Israel)
- Mylan NV, (US.)
- Novartis AG, (Switzerland)
- Pfizer Inc., (US.)
- Sun Pharmaceutical Industries Ltd., (India)
- Fresenius SE & Co. KGaA., (Germany)
- Lupin (India)
- Endo International plc., (Ireland)
- Aurobindo Pharma (India)
- Novartis AG (Switzerland)
- Hikma Pharmaceuticals PLC., (UK.)
- STADA Arzneimittel AG (Germany)
- Eli Lilly and Company (US)
- Aspen Holdings (South Africa)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL GENERIC DRUGS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL GENERIC DRUGS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL GENERIC DRUGS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUGS ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATEINT TREATMENT SUCCESS RATES
7 INDUSTRY INSIGHTS
7.1 PATENT ANALYSIS
7.2 DRUGS TREATMENT RATE BY MATURED MARKETS
7.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
7.4 PATIENT FLOW DIAGRAM
7.5 KEY PRICING STRATEGIES
7.6 KEY PATIENT ENROLLMENT STRATEGIES
7.7 INTERVIEWS WITH FORMULATION CHEMIST
7.8 OTHER KOL SNAPSHOTS
8 PIPELINE ANALYSIS
8.1 CLINICAL TRIALS AND PHASE ANALYSIS
8.2 DRUGS THERAPY PIPELINE
8.3 PHASE III CANDIDATES
8.4 PHASE II CANDIDATES
8.5 PHASE I CANDIDATES
8.6 OTHERS (PRE-CLINICAL AND RESEARCH)
9 REGULATORY FRAMEWORK
10 GLOBAL GENERIC DRUGS MARKET, BY TYPE
10.1 OVERVIEW
10.2 SIMPLE GENERICS
10.3 SUPER GENERICS
11 GLOBAL GENERIC DRUGS MARKET, BY BRAND
11.1 OVERVIEW
11.2 PURE GENERIC
11.3 BRANDED GENERIC
12 GLOBAL GENERIC DRUGS MARKET, BY INDICATION
(NOTE: MARKET VALUE, VOLUME AND ASP ANALYSIS WOULD BE PROVIDED FOR ALL SEGMENTS AND SUBSEGMENTS OF INDICATION)
12.1 OVERVIEW
12.2 CANCER DRUGS
12.2.1 ANTITUMOR ANTIBIOTICS
12.2.1.1. DAUNORUBICIN
12.2.1.1.1. MARKET VALUE(USD MILLION)
12.2.1.1.2. MARKET VOLUME(UNITS)
12.2.1.1.3. AVERAGE SELLING PRICE(ASP)
12.2.1.2. DOXORUBICIN
12.2.1.3. IDARUBICIN
12.2.1.4. MITOXANTRONE
12.2.1.5. OTHERS
12.2.2 DNA-DAMAGING AGENTS
12.2.2.1. CHLORAMBUCIL
12.2.2.2. CYCLOPHOSPHAMIDE
12.2.2.3. MELPHALAN
12.2.2.4. CARBOPLATIN
12.2.2.5. OTHERS
12.2.3 ANTIMETABOLITES
12.2.3.1. METHOTREXATE
12.2.3.2. FLUDARABINE
12.2.3.3. CYTARABINE
12.2.3.4. OTHERS
12.2.4 DNA-REPAIR ENZYME INHIBITORS
12.2.4.1. ETOPOSIDE
12.2.4.2. TOPOTECAN
12.2.4.3. OTHERS
12.2.5 ANTIMITOTIC DRUGS
12.2.5.1. VINCRISTINE
12.2.5.2. VINBLASTINE
12.2.5.3. OTHERS
12.2.6 OTHERS
12.3 CENTRAL NERVOUS SYSTEM PRODUCT
12.3.1 CHOLINESTERASE INHIBITORS
12.3.1.1. RIVASTIGMINE
12.3.1.2. DONEPEZIL
12.3.1.3. GALANTAMINE
12.3.1.4. TACRINE
12.3.1.5. OTHERS
12.3.2 NMDA RECEPTOR ANTAGONISTS
12.3.2.1. KETAMINE
12.3.2.2. DEXTROMETHORPHAN
12.3.2.3. MEMANTINE
12.3.2.4. AMANTADINE
12.3.3 ANTIEPILEPTIC
12.3.3.1. FIRST GENERATION
12.3.3.1.1. PHENOBARBITAL
12.3.3.1.2. PHENYTOIN
12.3.3.1.3. PRIMIDONE
12.3.3.1.4. ETHOSUXIMIDE
12.3.3.1.5. CARBAMAZEPINE
12.3.3.1.6. CLOBAZAM
12.3.3.2. SECOND GENERATION
12.3.3.2.1. VIGABATRIN
12.3.3.2.2. LAMOTRIGINE
12.3.3.2.3. GABAPENTIN
12.3.3.2.4. TOPIRAMATE
12.3.3.2.5. PREGABALIN
12.3.3.2.6. OTHERS
12.3.3.3. THIRD GENERATION
12.3.3.3.1. LACOSAMIDE
12.3.3.3.2. RUFINAMIDE
12.3.3.3.3. PERAMPANEL
12.3.3.3.4. OTHERS
12.3.4 ANTIPSYCHOTIC
12.3.4.1. TYPICAL ANTIPSYCHOTIC
12.3.4.1.1. HALOPERIDOL
12.3.4.1.2. LOXAPINE
12.3.4.1.3. THIORIDAZINE
12.3.4.1.4. MOLINDONE
12.3.4.1.5. THIOTHIXENE
12.3.4.1.6. FLUPHENAZINE
12.3.4.1.7. MESORIDAZINE
12.3.4.1.8. TRIFLUOPERAZINE
12.3.4.1.9. PERPHENAZINE
12.3.4.1.10. CHLORPROMAZINE
12.3.4.2. ATYPICAL ANTIPSYCHOTIC
12.3.4.2.1. ARIPIPRAZOLE
12.3.4.2.2. CLOZAPINE
12.3.4.2.3. ZIPRASIDONE
12.3.4.2.4. RISPERIDONE
12.3.4.2.5. QUETIAPINE
12.3.4.2.6. OLANZAPINE
12.3.5 OTHERS
12.4 DERMATOLOGICAL PRODUCTS
12.4.1 CORTICOSTEROIDS, BY TYPE
12.4.1.1. TOPICAL
12.4.1.1.1. MOMETASONE
12.4.1.1.2. BETAMETHASONE
12.4.1.1.3. HYDROCORTISONE
12.4.1.1.4. FLUTICASONE PROPIONATE
12.4.1.1.5. ALCLOMETASONE
12.4.1.1.6. TRIAMCINOLONE
12.4.1.1.7. FLUOCINOLONE ACETONIDE
12.4.1.1.8. CLOBETASOL PROPIONATE
12.4.1.1.9. OTHERS
12.4.1.2. ORAL
12.4.1.2.1. PREDNISOLONE
12.4.1.2.2. PREDNISONE
12.4.1.2.3. CORTISONE
12.4.1.2.4. METHYLPREDNISOLONE
12.4.1.2.5. OTHERS
12.4.2 RETINOIDS
12.4.2.1. ACITRETIN
12.4.2.2. ADAPALENE
12.4.2.3. ISOTRETINOIN
12.4.2.4. OTHERS
12.4.3 ANTIHISTAMINES AGENTS
12.4.3.1. CYPROHEPTADINE
12.4.3.2. DIPHENHYDRAMINE
12.4.3.3. HYDROXYZINE
12.4.3.4. OTHERS
12.4.4 CALCINEURIN INHIBITORS
12.4.4.1. TACROLIMUS
12.4.4.2. PIMECROLIMUS
12.4.5 ANTIINFECTIVES
12.4.5.1. ANTIBIOTICS
12.4.5.1.1. DOXYCYCLINE
12.4.5.1.2. RETAPAMULIN
12.4.5.1.3. DELAFLOXACIN
12.4.5.1.4. MINOCYCLINE
12.4.5.1.5. MUPIROCIN
12.4.5.1.6. OTHERS
12.4.5.2. ANTIFUNGAL
12.4.5.2.1. SYNTHETIC
12.4.5.2.1.1 CLOTRIMAZOLE
12.4.5.2.1.2 MICONAZOLE
12.4.5.2.1.3 OTHERS
12.4.5.2.2. IMIDAZOLES DRUGS
12.4.5.2.2.1 KETOCONAZOLE
12.4.5.2.2.2 CLONAZEPAM
12.4.5.2.3. OTHERS
12.4.5.2.4. OTHERS
12.4.5.3. ANTIVIRAL
12.4.5.3.1. ACYCLOVIR
12.4.5.3.2. FAMCICLOVIR
12.4.5.3.3. VALACYCLOVIR
12.4.5.3.4. OTHERS
12.4.5.4. OTHERS
12.4.6 HAIR GROWTH
12.4.6.1. MINOXIDIL
12.4.6.2. FINASTERIDE
12.4.6.3. SPIRONOLACTONE
12.4.6.4. DUTASTERIDE
12.4.6.5. OTHERS
12.4.7 OTHERS
12.5 GASTROINTESTINAL PRODUCTS
12.5.1 LAXATIVES
12.5.1.1. OSMOTIC LAXATIVES
12.5.1.1.1. GOLYTELY
12.5.1.1.2. COLYTE
12.5.1.1.3. MACROGOL 400
12.5.1.2. STIMULANT LAXATIVES
12.5.1.2.1. BISACODYL
12.5.1.2.2. CASTOR OIL
12.5.1.2.3. PHENOLPHTHALEIN
12.5.1.2.4. SENNA
12.5.1.3. BULK LAXATIVES
12.5.1.3.1. PSYLLIUM
12.5.1.3.2. METHYL CELLULOSE
12.5.1.3.3. POLYCARBOPHIL
12.5.1.3.4. OTHERS
12.5.1.4. LUBRICANT & EMOLLIENT LAXATIVES
12.5.1.4.1. MINERAL OIL
12.5.1.4.2. GLYCERIN SUPPOSITORIES
12.5.1.4.3. OTHERS
12.5.2 ANTACIDS
12.5.2.1. SODIUM ANTACIDS
12.5.2.2. CALCIUM ANTACIDS
12.5.2.3. MAGNESIUM ANTACIDS
12.5.2.4. ALUMINIUM ANTACIDS
12.5.2.5. OTHERS
12.5.3 ANTIDIARRHEALS
12.5.3.1. DIPHENOXYLATE
12.5.3.2. LOPERAMIDE
12.5.3.3. CODEINE
12.5.3.4. OTHERS
12.5.4 H2 BLOCKERS
12.5.4.1. FAMOTIDINE
12.5.4.2. RANITIDINE
12.5.4.3. OTHERS
12.5.5 PROTON PUMP INHIBITORS
12.5.5.1. OMEPRAZOLE
12.5.5.2. LANSOPRAZOLE
12.5.5.3. OTHERS
12.5.6 BILE ACID SEQUESTRANTS
12.5.6.1. CHOLESTYRAMINE
12.5.6.2. COLESTIPOL
12.5.7 OTHERS
12.6 RESPIRATORY PRODUCT
12.6.1 BRONCHODILATORS
12.6.1.1. ALBUTEROL
12.6.1.2. LEVALBUTEROL
12.6.1.3. SALMETEROL
12.6.1.4. FORMOTEROL
12.6.1.5. OTHERS
12.6.2 CORTICOSTEROIDS
12.6.2.1. RACEMIC EPINEPHRINE
12.6.2.2. FLUTICASONE
12.6.2.3. BUDESONIDE
12.6.2.4. OTHERS
12.6.3 MAST CELL STABILIZERS
12.6.3.1. MOMETASONE FUROATE
12.6.3.2. NEDOCROMIL
12.6.3.3. OTHERS
12.6.4 LEUKOTRIENE RECEPTOR ANTAGONISTS
12.6.4.1. CROMOLYN SODIUM
12.6.4.2. OMALIZUMAB
12.6.4.3. OTHERS
12.6.5 ANTIHISTAMINES
12.6.5.1. ZAFIRLUKAST
12.6.5.2. MONTELUKAST
12.6.5.3. ZILEUTON
12.6.5.4. OTHERS
12.6.6 RESPIRATORY STIMULANTS
12.6.6.1. LORATIDINE
12.6.6.2. FEXOFENADINE
12.6.6.3. CETIRIZINE
12.6.6.4. EPINEPHRINE
12.6.6.5. OTHERS
12.6.7 PULMONARY SURFACTANTS
12.6.7.1. DOXAPRAM
12.6.7.2. THEOPHYLLINE
12.6.7.3. PROGESTERONE
12.6.7.4. CAFFEINE
12.6.7.5. OTHERS
12.6.8 OXYGEN ANTIMICROBIALS
12.6.8.1. COLFOSCERIL PALMITATE
12.6.8.2. BERACTANT
12.6.8.3. CALFACTANT
12.6.8.4. PORACTANT ALFA
12.6.8.5. OTHERS
12.6.9 OTHERS
12.7 OPHTHALMIC PRODUCTS
12.7.1 ARTIFICIAL TEARS
12.7.1.1. DEMULCENT
12.7.1.1.1. POLYETHYLENE GLYCOL (PEG)
12.7.1.1.2. PROPYLENE GLYCOL
12.7.1.1.3. GLYCERIN
12.7.1.1.4. POLYVINYL ALCOHOL (PVA)
12.7.1.1.5. OTHERS
12.7.1.2. EMOLLIENTS
12.7.1.2.1. PARAFFIN
12.7.1.2.2. ANHYDROUS LANOLIN
12.7.1.2.3. WHITE WAX
12.7.1.2.4. OTHERS
12.7.1.3. OTHERS
12.7.2 NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
12.7.2.1. BROMFENAC
12.7.2.2. KETOROLAC
12.7.2.3. DICLOFENAC
12.7.2.4. OTHERS
12.7.3 CORTICOSTEROID
12.7.3.1. PREDNISOLONE
12.7.3.2. LOTEPREDNOL
12.7.3.3. FLUOROMETHOLONE
12.7.3.4. OTHERS
12.7.4 BETA BLOCKERS
12.7.4.1. LEVOBUNOLOL
12.7.4.2. TIMOLOL
12.7.4.3. BETAXOLOL
12.7.4.4. OTHERS
12.7.5 PROSTAGLANDIN ANALOGS
12.7.5.1. BIMATOPROST
12.7.5.2. TRAVOPROST
12.7.5.3. LATANOPROST
12.7.5.4. OTHERS
12.7.6 CARBONIC ANHYDRASE INHIBITORS
12.7.6.1. BRINZOLAMIDE
12.7.6.2. DORZOLAMIDE
12.7.7 COMBINATION DRUGS
12.7.7.1. BRIMONIDINE/TIMOLOL
12.7.7.2. DORZOLAMIDE/TIMOLOL
12.7.7.3. PHENYLEPHRINE
12.7.7.4. PROPARACAINE
12.7.7.5. TROPICAMIDE
12.7.7.6. OTHERS
12.8 CARDIOVASCULAR DRUGS
12.8.1 ACE INHIBITORS
12.8.1.1. BENAZEPRIL
12.8.1.2. CAPTOPRIL
12.8.1.3. ENALAPRIL MALEATE
12.8.1.4. LISINOPRIL
12.8.1.5. OTHERS
12.8.2 ANGIOTENSIN II RECEPTOR ANTAGONISTS (ARBS)
12.8.2.1. CANDESARTAN CILEXETIL
12.8.2.2. EPROSARTAN MESYLATE
12.8.2.3. IRBESARTAN
12.8.2.4. LOSARTAN
12.8.2.5. OTHERS
12.8.3 ANTIARRHYTHMICS
12.8.3.1. AMIODARONE
12.8.3.2. DISOPYRAMIDE PHOSPHATE
12.8.3.3. DOFETILIDE
12.8.3.4. FLECAINIDE
12.8.3.5. MEXILETINE HCL
12.8.3.6. PROCAINAMIDE
12.8.3.7. OTHERS
12.8.4 ANTICOAGULANTS
12.8.4.1. NON-VKA ORAL ANTICOAGULANTS (NOACS)
12.8.4.1.1. RIVAROXABAN
12.8.4.1.2. EDOXABAN
12.8.4.1.3. APIXABAN
12.8.4.1.4. OTHERS
12.8.4.2. HEPARIN & LMWH
12.8.4.2.1. DALTEPARIN
12.8.4.2.2. ENOXAPARIN
12.8.4.2.3. TINZAPARIN
12.8.4.2.4. OTHERS
12.8.4.3. VITAMIN K ANTAGONIST
12.8.4.3.1. WARFARIN
12.8.4.3.2. PHENPROCOUMON
12.8.4.3.3. OTHERS
12.8.4.4. THROMBIN INHIBITORS
12.8.4.4.1. BIVALIRUDIN
12.8.4.4.2. ARGATROBAN
12.8.4.4.3. DABIGATRAN
12.8.4.4.4. OTHERS
12.8.4.5. OTHERS
12.8.5 PLATELET INHIBITORS
12.8.5.1. ASPIRIN
12.8.5.2. CILOSTAZOL
12.8.5.3. CLOPIDOGRIL BISULFATE
12.8.5.4. DIPYRAMIDAMOLE
12.8.5.5. OTHERS
12.8.6 ANTIHYPERTENSIVES
12.8.6.1. CLONIDINE HCL
12.8.6.2. DOXAZOSIN MESYLATE
12.8.6.3. HYDRALAZINE HCI
12.8.6.4. METHYLDOPA
12.8.6.5. MINOXIDIL
12.8.6.6. OTHERS
12.8.7 BETA BLOCKERS
12.8.7.1. ACEBUTOLOL HCL
12.8.7.2. ATENOLOL
12.8.7.3. BETAXOLOL
12.8.7.4. BISOPROLOL
12.8.7.5. CARVEDILOL
12.8.7.6. LABETALOL HCL
12.8.7.7. METOPROLOL
12.8.7.8. METOPROLOL
12.8.7.9. NADOLOL
12.8.7.10. OTHERS
12.8.8 CALCIUM CHANNEL BLOCKERS
12.8.8.1. DIHYDROPYRIDINES
12.8.8.1.1. AMLODIPINE BESYLATE
12.8.8.1.2. NIFEDIPINE
12.8.8.1.3. NIMODIPINE
12.8.8.1.4. NISOLDIPINE
12.8.8.1.5. NICARDIPINE HCL
12.8.8.2. NONDIHYDROPYRIDINES
12.8.8.2.1. DILTIAZEM HCL
12.8.8.2.2. VERAPAMIL HCL
12.8.9 DIURETICS
12.8.9.1. THIAZIDE DIURETICS
12.8.9.1.1. CHLORTHALIDONE
12.8.9.1.2. HYDROCHLOROTHIAZIDE
12.8.9.1.3. METOLAZONE
12.8.9.1.4. INDAPAMIDE
12.8.9.2. LOOP DIURETICS
12.8.9.2.1. TORSEMIDE
12.8.9.2.2. FUROSEMIDE
12.8.9.2.3. BUMETANIDE
12.8.9.3. POTASSIUM-SPARING DIURETICS
12.8.9.3.1. AMILORIDE
12.8.9.3.2. TRIAMTERENE
12.8.9.3.3. SPIRONOLACTONE
12.8.9.3.4. EPLERENONE
12.8.9.4. OTHERS
12.8.10 LIPID MEDICATIONS
12.8.10.1. STATINS
12.8.10.1.1. ATORVASTATIN CALCIUM
12.8.10.1.2. FLUVASTATIN SODIUM
12.8.10.1.3. LOVASTATIN
12.8.10.1.4. OTHERS
12.8.10.2. FIBRATES
12.8.10.2.1. FENOFIBRATE
12.8.10.2.2. GEMFIBROZIL
12.8.10.3. BILE ACID SEQUESTRANTS
12.8.10.3.1. COLESEVELAM HCL
12.8.10.3.2. CHOLESTYRAMINE
12.8.10.3.3. COLESTIPOL HCL
12.8.10.4. OTHER LIPID MEDICATIONS
12.8.11 NITRATES
12.8.11.1. ORAL NITROGLYCERIN
12.8.11.2. NITROGLYCERIN OINTMENT
12.8.11.3. NITROGLYCERIN SKIN PATCHES
12.8.11.4. NITROGLYCERIN SUBLINGUAL TABLETS
12.8.11.5. OTHER NITROGLYCERIN TABLETS, CAPSULES, AND SPRAYS
12.8.12 OTHERS
12.9 VITAMIN
12.9.1 VITAMIN B
12.9.2 VITAMIN E
12.9.3 VITAMIN D
12.9.4 VITAMIN C
12.9.5 VITAMIN A
12.9.6 VITAMIN K
12.1 MINERALS
12.10.1 CALCIUM
12.10.2 MAGNESIUM
12.10.3 IRON
12.10.4 POTASSIUM
12.10.5 ZINC
12.10.6 CHROMIUM
12.10.7 SELENIUM
12.10.8 OTHERS
12.11 OTHERS
13 GLOBAL GENERIC DRUGS MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 ORAL
13.2.1 SOLID
13.2.1.1. SOLID, BY TYPE
13.2.1.1.1. TABLETS
13.2.1.1.2. CAPSULES
13.2.1.1.3. POWDER
13.2.1.1.4. PILLS
13.2.1.1.5. OTHERS
13.2.1.2. SOLID , BY DOSE
13.2.1.2.1. LESS THAN 100 MG
13.2.1.2.2. 100- 5OO MG
13.2.1.2.3. 500 MG – 1000 MG
13.2.1.2.4. MORE THAN 1000 MG
13.2.2 SEMI-SOLID
13.2.2.1. SEMI-SOLID, BY TYPE
13.2.2.1.1. GELS
13.2.2.1.2. EMULSIONS
13.2.2.1.3. ELIXIRS
13.2.2.1.4. OTHERS
13.2.2.2. SEMI-SOLID, BY DOSE
13.2.2.2.1. LESS THAN 25 ML
13.2.2.2.2. 25-50 ML
13.2.2.2.3. MORE THAN 50 ML
13.2.3 LIQUID
13.2.3.1. LIQUID, BY TYPE
13.2.3.1.1. SOLUTIONS
13.2.3.1.2. SYRUPS
13.2.3.1.3. OTHERS
13.2.3.2. LIQUID, BY DOSE
13.2.3.2.1. LESS THEN 25 ML
13.2.3.2.2. 25-50 ML
13.2.3.2.3. MORE THAN 50 ML
13.3 TOPICAL
13.3.1 LIQUID
13.3.1.1. LIQUID, BY TYPE
13.3.1.1.1. SOLUTIONS
13.3.1.1.2. SUSPENSIONS
13.3.1.2. LIQUID, BY DOSE
13.3.1.2.1. LESS THEN 25 ML
13.3.1.2.2. 25-50 ML
13.3.1.2.3. MORE THAN 50 ML
13.3.2 SEMI-SOLID
13.3.2.1. SEMI-SOLID, BY TYPE
13.3.2.1.1. CREAM
13.3.2.1.2. OINTMENT
13.3.2.1.3. GELS
13.3.2.1.4. PASTES
13.3.2.1.5. LOTIONS
13.3.2.1.6. OTHERS
13.3.2.2. SEMI-SOLID, BY DOSE
13.3.2.2.1. LESS THAN 25 MG
13.3.2.2.2. 25-50 MG
13.3.2.2.3. MORE THAN 50 MG
13.3.3 SOLID
13.3.3.1. SOLID, BY TYPE
13.3.3.1.1. SUPPOSITORIES
13.3.3.1.2. POWDERS
13.3.3.2. SOLID, BY DOSE
13.3.3.2.1. LESS THAN 1GM
13.3.3.2.2. 1GM
13.3.3.2.3. MORE THAN 1GM
13.3.4 OTHERS
13.4 PARENTERAL
13.4.1 PARENTERAL, BY TYPE
13.4.1.1. CONVENTIONAL DRUGS DELIVERY FORMULATIONS
13.4.1.1.1. SOLUTIONS
13.4.1.1.2. RECONSTITUTED/LYOPHILIZED
13.4.1.1.3. SUSPENSIONS
13.4.1.1.4. EMULSIONS
13.4.1.1.5. OTHERS
13.4.1.2. NOVEL DRUGS DELIVERY FORMULATIONS
13.4.1.2.1. COLLOIDAL DISPERSIONS
13.4.1.2.2. MICROPARTICLES
13.4.1.2.3. LONG ACTING INJECTION FORMULATION
13.4.2 PARENTERAL, BY DOSE
13.4.2.1. 1 MG/ML
13.4.2.2. 1-5 MG/ML
13.4.2.3. MORE THAN 5 MG/ML
13.5 OCCULAR
13.5.1 OCCULAR, BY TYPE
13.5.1.1. LIQUID
13.5.1.1.1. EYE DROPS
13.5.1.1.2. SPRAYS
13.5.1.2. SEMI-SOLID
13.5.1.2.1. GELS
13.5.1.2.2. OINTMENTS
13.5.2 OCCULAR, BY DOSE
13.5.2.1. 2.5ML
13.5.2.2. 5ML
13.5.2.3. 10 ML
13.6 INTRANASAL
13.6.1 INTRANASAL, BY TYPE
13.6.1.1. DROPS
13.6.1.2. SPRAYS
13.6.1.3. POWDERS
13.6.1.4. GELS
13.6.1.5. OTHERS
13.6.2 INTRANASAL, BY DOSE
13.6.2.1. 10 ML
13.6.2.2. 20 ML
13.6.3 OTHERS
13.6.4
14 GLOBAL GENERIC DRUGS MARKET, BY POPULATION TYPE
14.1 OVERVIEW
14.2 PEDIATRIC
14.3 ADULT
14.4 GERIATRIC
15 GLOBAL GENERIC DRUGS MARKET, BY MODE OF PURCHASE
15.1 OVERVIEW
15.2 OVER THE COUNTER
15.3 PRESCRIPTION
16 GLOBAL GENERIC DRUGS MARKET, BY END USER
16.1 OVERVIEW
16.2 HOSPITALS
16.2.1 PRIVATE
16.2.2 PUBLIC
16.3 SPECIALTY CLINICS
16.4 HOME HEALTHCARE
16.5 AMBULATORY SURGICAL CENTERS
16.6 COMMUNITY CENTRE
16.7 OTHERS
17 GLOBAL GENERIC DRUGS MARKET, BY DISTRIBUTION CHANNEL
17.1 OVERVIEW
17.2 DIRECT TENDER
17.3 RETAIL SALES
17.3.1 HOSPITAL PHARMACIES
17.3.2 RETAIL PHARMACIES
17.3.3 OTHER
17.4 ONLINE PHARMACIES
17.5 OTHER
18 GLOBAL GENERIC DRUGS MARKET, COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
18.2 COMPANY SHARE ANALYSIS: EUROPE
18.3 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
18.4 COMPANY SHARE ANALYSIS: SOUTH AMERICA
18.5 MERGERS & ACQUISITIONS
18.6 NEW PRODUCT DEVELOPMENT & APPROVALS
18.7 EXPANSIONS
18.8 REGULATORY CHANGES
18.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
19 GLOBAL GENERIC DRUGS MARKET, BY REGION
19.1 GLOBAL GENERIC DRUGS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
19.2 NORTH AMERICA
19.2.1 U.S.
19.2.2 CANADA
19.2.3 MEXICO
19.3 EUROPE
19.3.1 GERMANY
19.3.2 U.K.
19.3.3 ITALY
19.3.4 FRANCE
19.3.5 SPAIN
19.3.6 RUSSIA
19.3.7 SWITZERLAND
19.3.8 TURKEY
19.3.9 BELGIUM
19.3.10 NETHERLANDS
19.3.11 DENMARK
19.3.12 SWEDEN
19.3.13 POLAND
19.3.14 NORWAY
19.3.15 FINLAND
19.3.16 REST OF EUROPE
19.4 ASIA-PACIFIC
19.4.1 JAPAN
19.4.2 CHINA
19.4.3 SOUTH KOREA
19.4.4 INDIA
19.4.5 SINGAPORE
19.4.6 THAILAND
19.4.7 INDONESIA
19.4.8 MALAYSIA
19.4.9 PHILIPPINES
19.4.10 AUSTRALIA
19.4.11 NEW ZEALAND
19.4.12 VIETNAM
19.4.13 TAIWAN
19.4.14 REST OF ASIA-PACIFIC
19.5 SOUTH AMERICA
19.5.1 BRAZIL
19.5.2 ARGENTINA
19.5.3 REST OF SOUTH AMERICA
19.6 MIDDLE EAST AND AFRICA
19.6.1 SOUTH AFRICA
19.6.2 EGYPT
19.6.3 BAHRAIN
19.6.4 UNITED ARAB EMIRATES
19.6.5 KUWAIT
19.6.6 OMAN
19.6.7 QATAR
19.6.8 SAUDI ARABIA
19.6.9 REST OF MEA
19.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
20 GLOBAL GENERIC DRUGS MARKET, COMPANY PROFILE
20.1 BAYER
20.1.1 COMPANY OVERVIEW
20.1.2 REVENUE ANALYSIS
20.1.3 GEOGRAPHIC PRESENCE
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPEMENTS
20.2 SANOFI
20.2.1 COMPANY OVERVIEW
20.2.2 REVENUE ANALYSIS
20.2.3 GEOGRAPHIC PRESENCE
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPEMENTS
20.3 PFIZER, INC.
20.3.1 COMPANY OVERVIEW
20.3.2 REVENUE ANALYSIS
20.3.3 GEOGRAPHIC PRESENCE
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPEMENTS
20.4 GLAXOSMITHKLINE PLC
20.4.1 COMPANY OVERVIEW
20.4.2 REVENUE ANALYSIS
20.4.3 GEOGRAPHIC PRESENCE
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPEMENTS
20.5 TAKEDA PHARMACEUTICAL COMPANY LTD
20.5.1 COMPANY OVERVIEW
20.5.2 REVENUE ANALYSIS
20.5.3 GEOGRAPHIC PRESENCE
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPEMENTS
20.6 ABBOTT
20.6.1 COMPANY OVERVIEW
20.6.2 REVENUE ANALYSIS
20.6.3 GEOGRAPHIC PRESENCE
20.6.4 PRODUCT PORTFOLIO
20.6.5 RECENT DEVELOPEMENTS
20.7 NOVARTIS AG
20.7.1 COMPANY OVERVIEW
20.7.2 REVENUE ANALYSIS
20.7.3 GEOGRAPHIC PRESENCE
20.7.4 PRODUCT PORTFOLIO
20.7.5 RECENT DEVELOPEMENTS
20.8 SUN PHARMACEUTICAL INDUSTRIES
20.8.1 COMPANY OVERVIEW
20.8.2 REVENUE ANALYSIS
20.8.3 GEOGRAPHIC PRESENCE
20.8.4 PRODUCT PORTFOLIO
20.8.5 RECENT DEVELOPEMENTS
20.9 TEVA PHARMACEUTICAL INDUSTRIES LTD.
20.9.1 COMPANY OVERVIEW
20.9.2 REVENUE ANALYSIS
20.9.3 GEOGRAPHIC PRESENCE
20.9.4 PRODUCT PORTFOLIO
20.9.5 RECENT DEVELOPEMENTS
20.1 DR. REDDY’S LABORATORIES
20.10.1 COMPANY OVERVIEW
20.10.2 REVENUE ANALYSIS
20.10.3 GEOGRAPHIC PRESENCE
20.10.4 PRODUCT PORTFOLIO
20.10.5 RECENT DEVELOPEMENTS
20.11 ENDO PHARMACEUTICALS INC.
20.11.1 COMPANY OVERVIEW
20.11.2 REVENUE ANALYSIS
20.11.3 GEOGRAPHIC PRESENCE
20.11.4 PRODUCT PORTFOLIO
20.11.5 RECENT DEVELOPEMENTS
20.12 AMNEAL PHARMACEUTICALS INC
20.12.1 COMPANY OVERVIEW
20.12.2 REVENUE ANALYSIS
20.12.3 GEOGRAPHIC PRESENCE
20.12.4 PRODUCT PORTFOLIO
20.12.5 RECENT DEVELOPEMENTS
20.13 ALKEM LABORATORIES LTD
20.13.1 COMPANY OVERVIEW
20.13.2 REVENUE ANALYSIS
20.13.3 GEOGRAPHIC PRESENCE
20.13.4 PRODUCT PORTFOLIO
20.13.5 RECENT DEVELOPEMENTS
20.14 DAIICHI SANKYO COMPANY, LIMITED
20.14.1 COMPANY OVERVIEW
20.14.2 REVENUE ANALYSIS
20.14.3 GEOGRAPHIC PRESENCE
20.14.4 PRODUCT PORTFOLIO
20.14.5 RECENT DEVELOPEMENTS
20.15 MYLAN N.V. (VIATRIS INC.)
20.15.1 COMPANY OVERVIEW
20.15.2 REVENUE ANALYSIS
20.15.3 GEOGRAPHIC PRESENCE
20.15.4 PRODUCT PORTFOLIO
20.15.5 RECENT DEVELOPEMENTS
20.16 AUROBINDO PHARMA
20.16.1 COMPANY OVERVIEW
20.16.2 REVENUE ANALYSIS
20.16.3 GEOGRAPHIC PRESENCE
20.16.4 PRODUCT PORTFOLIO
20.16.5 RECENT DEVELOPEMENTS
20.17 ZYDUS PHARMACEUTICALS, INC.
20.17.1 COMPANY OVERVIEW
20.17.2 REVENUE ANALYSIS
20.17.3 GEOGRAPHIC PRESENCE
20.17.4 PRODUCT PORTFOLIO
20.17.5 RECENT DEVELOPEMENTS
20.18 LUPIN
20.18.1 COMPANY OVERVIEW
20.18.2 REVENUE ANALYSIS
20.18.3 GEOGRAPHIC PRESENCE
20.18.4 PRODUCT PORTFOLIO
20.18.5 RECENT DEVELOPEMENTS
20.19 SANDOZ INTERNATIONAL GMBH
20.19.1 COMPANY OVERVIEW
20.19.2 REVENUE ANALYSIS
20.19.3 GEOGRAPHIC PRESENCE
20.19.4 PRODUCT PORTFOLIO
20.19.5 RECENT DEVELOPEMENTS
20.2 FRESENIUS MEDICAL CARE AG & CO. KGAA
20.20.1 COMPANY OVERVIEW
20.20.2 REVENUE ANALYSIS
20.20.3 GEOGRAPHIC PRESENCE
20.20.4 PRODUCT PORTFOLIO
20.20.5 RECENT DEVELOPEMENTS
20.21 SANOFI
20.21.1 COMPANY OVERVIEW
20.21.2 REVENUE ANALYSIS
20.21.3 GEOGRAPHIC PRESENCE
20.21.4 PRODUCT PORTFOLIO
20.21.5 RECENT DEVELOPEMENTS
20.22 CIPLA INC.
20.22.1 COMPANY OVERVIEW
20.22.2 REVENUE ANALYSIS
20.22.3 GEOGRAPHIC PRESENCE
20.22.4 PRODUCT PORTFOLIO
20.22.5 RECENT DEVELOPEMENTS
20.23 ASTRAZENECA
20.23.1 COMPANY OVERVIEW
20.23.2 REVENUE ANALYSIS
20.23.3 GEOGRAPHIC PRESENCE
20.23.4 PRODUCT PORTFOLIO
20.23.5 RECENT DEVELOPEMENTS
20.24 BRISTOL-MYERS SQUIBB
20.24.1 COMPANY OVERVIEW
20.24.2 REVENUE ANALYSIS
20.24.3 GEOGRAPHIC PRESENCE
20.24.4 PRODUCT PORTFOLIO
20.24.5 RECENT DEVELOPEMENTS
20.25 PAR PHARMACEUTICALS
20.25.1 COMPANY OVERVIEW
20.25.2 REVENUE ANALYSIS
20.25.3 GEOGRAPHIC PRESENCE
20.25.4 PRODUCT PORTFOLIO
20.25.5 RECENT DEVELOPEMENTS
20.26 HIKMA PHARMACEUTICALS PLC
20.26.1 COMPANY OVERVIEW
20.26.2 REVENUE ANALYSIS
20.26.3 GEOGRAPHIC PRESENCE
20.26.4 PRODUCT PORTFOLIO
20.26.5 RECENT DEVELOPEMENTS
20.27 RECKITT BENCKISER
20.27.1 COMPANY OVERVIEW
20.27.2 REVENUE ANALYSIS
20.27.3 GEOGRAPHIC PRESENCE
20.27.4 PRODUCT PORTFOLIO
20.27.5 RECENT DEVELOPEMENTS
20.28 PERRIGO
20.28.1 COMPANY OVERVIEW
20.28.2 REVENUE ANALYSIS
20.28.3 GEOGRAPHIC PRESENCE
20.28.4 PRODUCT PORTFOLIO
20.28.5 RECENT DEVELOPEMENTS
20.29 TAISHO PHARMACEUTICAL
20.29.1 COMPANY OVERVIEW
20.29.2 REVENUE ANALYSIS
20.29.3 GEOGRAPHIC PRESENCE
20.29.4 PRODUCT PORTFOLIO
20.29.5 RECENT DEVELOPEMENTS
20.3 MALLINCKRODT
20.30.1 COMPANY OVERVIEW
20.30.2 REVENUE ANALYSIS
20.30.3 GEOGRAPHIC PRESENCE
20.30.4 PRODUCT PORTFOLIO
20.30.5 RECENT DEVELOPEMENTS
20.31 AMGEN, INC.
20.31.1 COMPANY OVERVIEW
20.31.2 REVENUE ANALYSIS
20.31.3 GEOGRAPHIC PRESENCE
20.31.4 PRODUCT PORTFOLIO
20.31.5 RECENT DEVELOPEMENTS
20.32 ARENA PHARMACEUTICALS, INC.
20.32.1 COMPANY OVERVIEW
20.32.2 REVENUE ANALYSIS
20.32.3 GEOGRAPHIC PRESENCE
20.32.4 PRODUCT PORTFOLIO
20.32.5 RECENT DEVELOPEMENTS
20.33 STADA ARZNEIMITTEL AG
20.33.1 COMPANY OVERVIEW
20.33.2 REVENUE ANALYSIS
20.33.3 GEOGRAPHIC PRESENCE
20.33.4 PRODUCT PORTFOLIO
20.33.5 RECENT DEVELOPEMENTS
20.34 ACCORD HEALTHCARE GMBH
20.34.1 COMPANY OVERVIEW
20.34.2 REVENUE ANALYSIS
20.34.3 GEOGRAPHIC PRESENCE
20.34.4 PRODUCT PORTFOLIO
20.34.5 RECENT DEVELOPEMENTS
20.35 ASCENDIS PHARMA GROUP
20.35.1 COMPANY OVERVIEW
20.35.2 REVENUE ANALYSIS
20.35.3 GEOGRAPHIC PRESENCE
20.35.4 PRODUCT PORTFOLIO
20.35.5 RECENT DEVELOPEMENTS
20.36 ALVOGEN
20.36.1 COMPANY OVERVIEW
20.36.2 REVENUE ANALYSIS
20.36.3 GEOGRAPHIC PRESENCE
20.36.4 PRODUCT PORTFOLIO
20.36.5 RECENT DEVELOPEMENTS
20.37 ANI PHARMACEUTICALS, INC.
20.37.1 COMPANY OVERVIEW
20.37.2 REVENUE ANALYSIS
20.37.3 GEOGRAPHIC PRESENCE
20.37.4 PRODUCT PORTFOLIO
20.37.5 RECENT DEVELOPEMENTS
20.38 ACELLA PHARMACEUTICALS, LLC
20.38.1 COMPANY OVERVIEW
20.38.2 REVENUE ANALYSIS
20.38.3 GEOGRAPHIC PRESENCE
20.38.4 PRODUCT PORTFOLIO
20.38.5 RECENT DEVELOPEMENTS
20.39 GLENMARK PHARMACEUTICALS
20.39.1 COMPANY OVERVIEW
20.39.2 REVENUE ANALYSIS
20.39.3 GEOGRAPHIC PRESENCE
20.39.4 PRODUCT PORTFOLIO
20.39.5 RECENT DEVELOPEMENTS
20.4 HORIZON THERAPEUTICS PLC
20.40.1 COMPANY OVERVIEW
20.40.2 REVENUE ANALYSIS
20.40.3 GEOGRAPHIC PRESENCE
20.40.4 PRODUCT PORTFOLIO
20.40.5 RECENT DEVELOPEMENTS
20.41 SANIS
20.41.1 COMPANY OVERVIEW
20.41.2 REVENUE ANALYSIS
20.41.3 GEOGRAPHIC PRESENCE
20.41.4 PRODUCT PORTFOLIO
20.41.5 RECENT DEVELOPEMENTS
20.42 MAYNE PHARMA
20.42.1 COMPANY OVERVIEW
20.42.2 REVENUE ANALYSIS
20.42.3 GEOGRAPHIC PRESENCE
20.42.4 PRODUCT PORTFOLIO
20.42.5 RECENT DEVELOPEMENTS
20.43 OTSUKA PHARMACEUTICALS
20.43.1 COMPANY OVERVIEW
20.43.2 REVENUE ANALYSIS
20.43.3 GEOGRAPHIC PRESENCE
20.43.4 PRODUCT PORTFOLIO
20.43.5 RECENT DEVELOPEMENTS
20.44 WACKHARDT
20.44.1 COMPANY OVERVIEW
20.44.2 REVENUE ANALYSIS
20.44.3 GEOGRAPHIC PRESENCE
20.44.4 PRODUCT PORTFOLIO
20.44.5 RECENT DEVELOPEMENTS
20.45 TORQUE PHARMACEUTICALS PVT. LTD
20.45.1 COMPANY OVERVIEW
20.45.2 REVENUE ANALYSIS
20.45.3 GEOGRAPHIC PRESENCE
20.45.4 PRODUCT PORTFOLIO
20.45.5 RECENT DEVELOPEMENTS
20.46 JIANGSU HENGRUI PHARMACEUTICALS CO., LTD.
20.46.1 COMPANY OVERVIEW
20.46.2 REVENUE ANALYSIS
20.46.3 GEOGRAPHIC PRESENCE
20.46.4 PRODUCT PORTFOLIO
20.46.5 RECENT DEVELOPEMENTS
20.47 KRKA LTD
20.47.1 COMPANY OVERVIEW
20.47.2 REVENUE ANALYSIS
20.47.3 GEOGRAPHIC PRESENCE
20.47.4 PRODUCT PORTFOLIO
20.47.5 RECENT DEVELOPEMENTS
20.48 HYPERA PHARMA
20.48.1 COMPANY OVERVIEW
20.48.2 REVENUE ANALYSIS
20.48.3 GEOGRAPHIC PRESENCE
20.48.4 PRODUCT PORTFOLIO
20.48.5 RECENT DEVELOPEMENTS
20.49 HEBEI TIANCHENG PHARMACEUTICAL COMPANY LTD
20.49.1 COMPANY OVERVIEW
20.49.2 REVENUE ANALYSIS
20.49.3 GEOGRAPHIC PRESENCE
20.49.4 PRODUCT PORTFOLIO
20.49.5 RECENT DEVELOPEMENTS
20.5 POLY PHARMACEUTICALS, INC.
20.50.1 COMPANY OVERVIEW
20.50.2 REVENUE ANALYSIS
20.50.3 GEOGRAPHIC PRESENCE
20.50.4 PRODUCT PORTFOLIO
20.50.5 RECENT DEVELOPEMENTS
21 RELATED REPORTS
22 CONCLUSION
23 QUESTIONNAIRE
24 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.