Global Her2 Positive Breast Cancer Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
10.50 Billion
USD
11.83 Billion
2021
2029
USD
10.50 Billion
USD
11.83 Billion
2021
2029
| 2022 –2029 | |
| USD 10.50 Billion | |
| USD 11.83 Billion | |
|
|
|
|
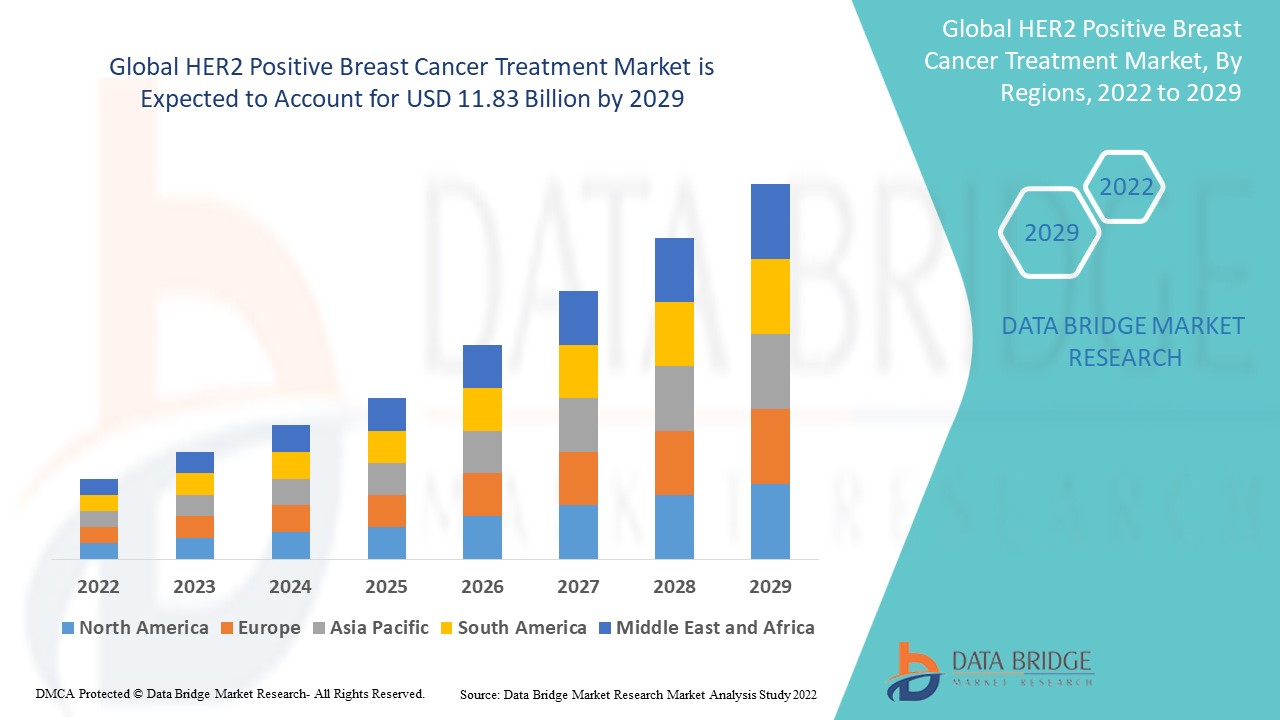
HER2 Positive Breast Cancer Treatment Market Analysis and Size
The global HER2 positive breast cancer treatment market is expected to witness significant growth during the forecast period. Breast cancer is the second most common cancer in the world and is the most common cancer in women globally. Human epidermal growth factor receptor 2-positive (HER2+) breast cancer consist of around 20% of breast cancer cases and was associated with poor prognosis in the absence of several effective treatments. The endlessly improving healthcare infrastructure, along with the increase in disposable incomes of people in Asia-Pacific region is expected to make the market grow in the forecast period. COVID-19 also had a major impact on the market growth.
Data Bridge Market Research analyses a growth rate in the global HER2 positive breast cancer treatment market in the forecast period 2022-2029. The expected CAGR of global HER2 positive breast cancer treatment market is tend to be around 1.50% in the mentioned forecast period. The market was valued at USD 10.50 billion in 2021, and it would grow upto USD 11.83 billion by 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
HER2 Positive Breast Cancer Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Treatment Type (Chemotherapy, Targeted Therapy Immunotherapy, Others), Route of Administration (Oral, Parenteral, Others), End-Users (Hospitals, Homecare, Speciality Centres, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Johnson & Johnson Private Limited (U.S.), Cipla Inc. (U.S.), Abbott (U.S.), AbbVie Inc. (U.S.), Merck KGaA (Germany), Sun Pharmaceutical Industries Ltd. (India), Aurobindo Pharma (India), Lupin (India), Hikma Pharmaceuticals PLC (U.K.), Amneal Pharmaceuticals LLC. (U.S.), Pfizer Inc (U.S.), Mylan N.V. (U.S.), Novartis AG (Switzerland), Bristol-Myers Squibb Company (U.S.), GSK plc. (U.K.), Bayer AG (Germany) |
|
Market Opportunities |
|
Market Definition
HER2-positive breast cancer treatment is a type of breast cancer wherein the test confirmed the presence of human epidermal growth factor receptor 2 (HER2), a protein that boosts cancer cells' growth. Breast cancer is not a single disease but a group of numerous different tumor subtypes. Although many subtypes exist, they are usually categorized by the presence or absence of hormonal receptors (HRs), which includes estrogen receptor (ER) and the progesterone receptor (PR), along with human epidermal growth factor receptor 2 (HER2). It is of great importance to the healthcare sector and thus is expected to rise high in the forecast period.
Global HER2 Positive Breast Cancer Treatment Market Dynamics
Drivers
- Increase in HER2 Positive Breast Cancer
The rate of HER2-positive (HR+/HER2+ and HR–/HER2+) breast cancer rise from 10–11 in 2020 to 13–14 per 100,000 women in 2030. This boost the market growth.
- Rising Demand for Oral Drugs
Oral drugs is expected to boost the market growth. The segment is expected to accelerate the global esophageal cancer market as most products are available in capsule and tablet form and it is a very feasible route of administration.
Opportunities
- Increased Drug Launches
Several drug launches are associated with the creation of opportunities for he market. Approval and launch of premium-priced therapeutics during the forecast period such as two small molecule inhibitors (Ibrance and Piqray), two antibody-drug conjugates (trastuzumab duocarmazine and disitamab vedotin), and a checkpoint inhibitor (Tecentriq) Label expansion of established drugs like Enhertu and Tukysa are benefitting the market growth.
- Higher Indulgence of Immunotherapy
The segment of immunotherapy is projected to gain further attraction during the forecast period. Immunotherapeutics tend to have less adverse effects when compared to chemotherapeutics. Non-specificity, toxic side effects, and development of resistance associated with chemotherapy are most likely to suppress the growth of chemotherapeutics and boost the adoption of immunotherapeutics and other targeted treatments. This creates more opportunity in the market.
Restraints/Challenges
- Lack of Skilled Professionals
Lack of trained professionals who are not aware of the knowledge of the appropriate treatment methods for this disease could restraint the growth of the global HER2 positive breast cancer treatment market over a forecast period.
- Patent Expiration of the Drugs
The patent expiration of several drugs hamper the market growth. Patent expiries of the top brands particularly Perjeta, Herceptin and Kadcyla, which held the majority of sales in 2020 are hampering the market growth.
This global HER2 positive breast cancer treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the global HER2 positive breast cancer treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global HER2 Positive Breast Cancer Treatment Market Scope
The global HER2 positive breast cancer treatment market is segmented on the basis of treatment type, route of administration, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Treatment Type
- Immunotherapy
- Targeted Therapy
- Chemotherapy
- Others
Route of Administration
- Oral
- Parenteral
End-Users
- Hospitals
- Homecare
- Speciality Centres
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
HER2 Positive Breast Cancer Treatment Market Regional Analysis/Insights
The global HER2 positive breast cancer treatment market is analysed and market size insights and trends are provided by treatment type, route of administration, distribution channel and end-user as referenced above.
The major countries covered in the global HER2 positive breast cancer treatment market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is expected to have the highest market growth due to the rise cases of HER2-positive breast cancer, favourable government policies, and advanced healthcare facilities.
Asia-Pacific dominates the market due to the increase government initiatives and rapidly increasing disposable income.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Intestine Cancer Therapeutics Market Share Analysis
The global HER2 positive breast cancer treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to global HER2 positive breast cancer treatment market.
Key players operating in the global HER2 positive breast cancer treatment market include:
- Johnson & Johnson Private Limited (U.S.)
- Cipla Inc. (U.S.)
- Abbott (U.S.)
- AbbVie Inc. (U.S.)
- Merck KGaA (Germany)
- Sun Pharmaceutical Industries Ltd. (India)
- Aurobindo Pharma (India)
- Lupin (India)
- Hikma Pharmaceuticals PLC (U.K.)
- Amneal Pharmaceuticals LLC. (U.S.)
- Pfizer Inc (U.S.)
- Mylan N.V. (U.S.)
- Novartis AG (Switzerland)
- Bristol-Myers Squibb Company (U.S.)
- GSK plc. (U.K.)
- Bayer AG (Germany)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER'S 5 FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
5.1 DIAGNOSED INCIDENT CASES OF ALL INVASIVE BREAST CANCER
5.2 AGE-SPECIFIC DIAGNOSED INCIDENT CASES OF ALL INVASIVE BREAST CANCER
5.3 TREATMENT RATE
5.4 MORTALITY RATE
5.5 DRUG ADHERENCE AND THERAPY SWITCH MODEL
5.6 PATEINT TREATMENT SUCCESS RATES
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH OCBOLOGIST
6.8 INTERVIEWS WITH RESEARCHERS
6.9 OTHER KOL SNAPSHOTS
7 REGULATORY SCENARIO
7.1 FDA APPROVALS
7.2 EMA APPROVALS
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
10 MARKET OVERVIEW
10.1 DRIVERS
10.2 RESTRAINS
10.3 OPPURTUNITY
10.4 CHALLENGES
11 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY TREATMENT TYPE
11.1 OVERVIEW
11.2 CHEMOTHERAPY
11.2.1 TAXANES
11.2.1.1. BY DRUGS
11.2.1.1.1. PACLITAXEL
11.2.1.1.1.1 MARKET VALUE (USD MN)
11.2.1.1.1.2 MARKET VOLUME (IU)
11.2.1.1.1.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.2. DOCETAXEL
11.2.1.1.2.1 MARKET VALUE (USD MN)
11.2.1.1.2.2 MARKET VOLUME (IU)
11.2.1.1.2.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.3. ALBUMIN BOUND PACLITAXEL
11.2.1.1.3.1 MARKET VALUE (USD MN)
11.2.1.1.3.2 MARKET VOLUME (IU)
11.2.1.1.3.3 AVERAGE SELLING PRICE (USD)
11.2.1.1.4. OTHERS
11.2.1.2. BY DRUG TYPE
11.2.1.2.1. GENERICS
11.2.1.2.1.1 MARKET VALUE (USD MN)
11.2.1.2.1.2 MARKET VOLUME (IU)
11.2.1.2.1.3 AVERAGE SELLING PRICE (USD)
11.2.1.2.2. BRANDED
11.2.1.2.2.1 MARKET VALUE (USD MN)
11.2.1.2.2.2 MARKET VOLUME (IU)
11.2.1.2.2.3 AVERAGE SELLING PRICE (USD)
11.2.2 ANTHRACYCLINES
11.2.2.1. BY TYPE
11.2.2.1.1. DOXORUBICIN
11.2.2.1.1.1 MARKET VALUE (USD MN)
11.2.2.1.1.2 MARKET VOLUME (IU)
11.2.2.1.1.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.2. LIPOSOMAL DOXORUBICIN
11.2.2.1.2.1 MARKET VALUE (USD MN)
11.2.2.1.2.2 MARKET VOLUME (IU)
11.2.2.1.2.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.3. EPIRUBICIN
11.2.2.1.3.1 MARKET VALUE (USD MN)
11.2.2.1.3.2 MARKET VOLUME (IU)
11.2.2.1.3.3 AVERAGE SELLING PRICE (USD)
11.2.2.1.4. OTHERS
11.2.2.2. BY DRUG TYPE
11.2.2.2.1. GENERICS
11.2.2.2.1.1 MARKET VALUE (USD MN)
11.2.2.2.1.2 MARKET VOLUME (IU)
11.2.2.2.1.3 AVERAGE SELLING PRICE (USD)
11.2.2.2.2. BRANDED
11.2.2.2.2.1 MARKET VALUE (USD MN)
11.2.2.2.2.2 MARKET VOLUME (IU)
11.2.2.2.2.3 AVERAGE SELLING PRICE (USD)
11.2.3 PLATINUM AGENTS
11.2.3.1. BY TYPE
11.2.3.1.1. CISPLATIN
11.2.3.1.1.1 MARKET VALUE (USD MN)
11.2.3.1.1.2 MARKET VOLUME (IU)
11.2.3.1.1.3 AVERAGE SELLING PRICE (USD)
11.2.3.1.2. CARBOPLATIN
11.2.3.1.2.1 MARKET VALUE (USD MN)
11.2.3.1.2.2 MARKET VOLUME (IU)
11.2.3.1.2.3 AVERAGE SELLING PRICE (USD)
11.2.3.1.3. OTHERS
11.2.3.2. BY DRUG TYPE
11.2.3.2.1. GENERICS
11.2.3.2.1.1 MARKET VALUE (USD MN)
11.2.3.2.1.2 MARKET VOLUME (IU)
11.2.3.2.1.3 AVERAGE SELLING PRICE (USD)
11.2.3.2.2. BRANDED
11.2.3.2.2.1 MARKET VALUE (USD MN)
11.2.3.2.2.2 MARKET VOLUME (IU)
11.2.3.2.2.3 AVERAGE SELLING PRICE (USD)
11.2.4 ANTIMETABOLITES
11.2.4.1. BY TYPE
11.2.4.1.1. CAPECITABINE
11.2.4.1.1.1 MARKET VALUE (USD MN)
11.2.4.1.1.2 MARKET VOLUME (IU)
11.2.4.1.1.3 AVERAGE SELLING PRICE (USD)
11.2.4.1.2. GEMCITABINE
11.2.4.1.2.1 MARKET VALUE (USD MN)
11.2.4.1.2.2 MARKET VOLUME (IU)
11.2.4.1.2.3 AVERAGE SELLING PRICE (USD)
11.2.4.2. BY DRUG TYPE
11.2.4.2.1. GENERICS
11.2.4.2.1.1 MARKET VALUE (USD MN)
11.2.4.2.1.2 MARKET VOLUME (IU)
11.2.4.2.1.3 AVERAGE SELLING PRICE (USD)
11.2.4.2.2. BRANDED
11.2.4.2.2.1 MARKET VALUE (USD MN)
11.2.4.2.2.2 MARKET VOLUME (IU)
11.2.4.2.2.3 AVERAGE SELLING PRICE (USD)
11.2.5 MICROTUBULE INHIBITORS
11.2.5.1. BY TYPE
11.2.5.1.1. IXABEPILONE
11.2.5.1.1.1 MARKET VALUE (USD MN)
11.2.5.1.1.2 MARKET VOLUME (IU)
11.2.5.1.1.3 AVERAGE SELLING PRICE (USD)
11.2.5.1.2. ERBULIN
11.2.5.1.2.1 MARKET VALUE (USD MN)
11.2.5.1.2.2 MARKET VOLUME (IU)
11.2.5.1.2.3 AVERAGE SELLING PRICE (USD)
11.2.5.1.3. OTHERS
11.2.5.2. BY DRUG TYPE
11.2.5.2.1. GENERICS
11.2.5.2.1.1 MARKET VALUE (USD MN)
11.2.5.2.1.2 MARKET VOLUME (IU)
11.2.5.2.1.3 AVERAGE SELLING PRICE (USD)
11.2.5.2.2. BRANDED
11.2.5.2.2.1 MARKET VALUE (USD MN)
11.2.5.2.2.2 MARKET VOLUME (IU)
11.2.5.2.2.3 AVERAGE SELLING PRICE (USD)
11.3 TARGETED DRUG THERAPY
11.3.1 MONOCLONAL ANTIBODIES
11.3.1.1. BY TYPE
11.3.1.1.1. TRASTUZUMAB
11.3.1.1.1.1 MARKET VALUE (USD MN)
11.3.1.1.1.2 MARKET VOLUME (IU)
11.3.1.1.1.3 AVERAGE SELLING PRICE (USD)
11.3.1.1.2. PERTUZUMAB
11.3.1.1.2.1 MARKET VALUE (USD MN)
11.3.1.1.2.2 MARKET VOLUME (IU)
11.3.1.1.2.3 AVERAGE SELLING PRICE (USD)
11.3.1.1.3. TRASTUZUMAB, PERTUZUMAB, AND HYALURONIDASE INJECTION (COMBINATION DRUGS)
11.3.1.1.3.1 MARKET VALUE (USD MN)
11.3.1.1.3.2 MARKET VOLUME (IU)
11.3.1.1.3.3 AVERAGE SELLING PRICE (USD)
11.3.1.1.4. MARGETUXIMAB
11.3.1.1.4.1 MARKET VALUE (USD MN)
11.3.1.1.4.2 MARKET VOLUME (IU)
11.3.1.1.4.3 AVERAGE SELLING PRICE (USD)
11.3.1.1.5. OTHERS
11.3.1.2. BY DRUG TYPE
11.3.1.2.1. BIOLOGICS
11.3.1.2.1.1 MARKET VALUE (USD MN)
11.3.1.2.1.2 MARKET VOLUME (IU)
11.3.1.2.1.3 AVERAGE SELLING PRICE (USD)
11.3.1.2.2. BIOSIMILAR
11.3.1.2.2.1 MARKET VALUE (USD MN)
11.3.1.2.2.2 MARKET VOLUME (IU)
11.3.1.2.2.3 AVERAGE SELLING PRICE (USD)
11.3.2 KINASE INHIBITORS
11.3.2.1. BY TYPE
11.3.2.1.1. TUCATINIB
11.3.2.1.1.1 MARKET VALUE (USD MN)
11.3.2.1.1.2 MARKET VOLUME (IU)
11.3.2.1.1.3 AVERAGE SELLING PRICE (USD)
11.3.2.1.2. LAPATINIB
11.3.2.1.2.1 MARKET VALUE (USD MN)
11.3.2.1.2.2 MARKET VOLUME (IU)
11.3.2.1.2.3 AVERAGE SELLING PRICE (USD)
11.3.2.1.3. NERTINIB
11.3.2.1.3.1 MARKET VALUE (USD MN)
11.3.2.1.3.2 MARKET VOLUME (IU)
11.3.2.1.3.3 AVERAGE SELLING PRICE (USD)
11.3.2.2. BY DRUG TYPE
11.3.2.2.1. GENERICS
11.3.2.2.1.1 MARKET VALUE (USD MN)
11.3.2.2.1.2 MARKET VOLUME (IU)
11.3.2.2.1.3 AVERAGE SELLING PRICE (USD)
11.3.2.2.2. BRANDED
11.3.2.2.2.1 MARKET VALUE (USD MN)
11.3.2.2.2.2 MARKET VOLUME (IU)
11.3.2.2.2.3 AVERAGE SELLING PRICE (USD)
11.3.3 ANTIBODY DRUG CONJUGATES
11.3.3.1. ADO-TRASTUZUMAB EMTANSINE
11.3.3.1.1. MARKET VALUE (USD MN)
11.3.3.1.2. MARKET VOLUME (IU)
11.3.3.1.3. AVERAGE SELLING PRICE (USD)
11.3.3.2. FAM-TRASTUZUMAB DERUXTECAN
11.3.3.2.1. MARKET VALUE (USD MN)
11.3.3.2.2. MARKET VOLUME (IU)
11.3.3.2.3. AVERAGE SELLING PRICE (USD)
11.3.3.3. OTHERS
11.4 HORMONE THERAPY
11.4.1 BY TYPE
11.4.1.1. TAMOXIFEN
11.4.1.1.1. MARKET VALUE (USD MN)
11.4.1.1.2. MARKET VOLUME (IU)
11.4.1.1.3. AVERAGE SELLING PRICE (USD)
11.4.1.2. TOREMIFENE
11.4.1.2.1. MARKET VALUE (USD MN)
11.4.1.2.2. MARKET VOLUME (IU)
11.4.1.2.3. AVERAGE SELLING PRICE (USD)
11.4.1.3. FULVESTRANT
11.4.1.3.1. MARKET VALUE (USD MN)
11.4.1.3.2. MARKET VOLUME (IU)
11.4.1.3.3. AVERAGE SELLING PRICE (USD)
11.4.1.4. OTHERS
11.4.2 BY DRUG TYPE
11.4.2.1. GENERICS
11.4.2.1.1. MARKET VALUE (USD MN)
11.4.2.1.2. MARKET VOLUME (IU)
11.4.2.1.3. AVERAGE SELLING PRICE (USD)
11.4.2.2. BRANDED
11.4.2.2.1. MARKET VALUE (USD MN)
11.4.2.2.2. MARKET VOLUME (IU)
11.4.2.2.3. AVERAGE SELLING PRICE (USD)
11.5 OTHERS
11.5.1 RADIATION THERAPY
11.5.2 SURGERY
11.5.3 RECONSTRUCTIVE SURGERY
12 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY USAGE TYPE
12.1 NEOADJUVENT USE
12.2 ADJUVANT USE
13 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY STAGES
13.1 STAGE 0
13.2 STAGE I
13.3 STAGE II
13.4 STAGE III
13.5 STAGE IV
14 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY AGE GROUP
14.1 OVERVIEW
14.2 BELOW 30 YEARS
14.3 30-50 YEARS
14.4 ABOVE 50 YEARS
15 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
15.1 OVERVIEW
15.2 ORAL
15.3 PARENTERAL
15.4 OTHERS
16 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY END USER
16.1 OVERVIEW
16.2 HOSPITALS
16.3 SPECIALTY CLINICS
16.4 ACADEMIC AND RESEARCH INSTITUTES
16.5 OTHERS
17 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY DISTRIBUTION CHANNEL
17.1 OVERVIEW
17.2 HOSPITAL PHARMACY
17.3 RETAIL PHARMACY
17.4 ONLINE PHARMACY
17.5 OTHERS
18 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY GEOGRAPHY
18.1 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
18.2 NORTH AMERICA
18.2.1 U.S.
18.2.1.1. U.S. HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY TREATMENT
18.2.1.2. U.S. HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY USAGE TYPE
18.2.1.3. U.S. HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY STAGES
18.2.1.4. U.S. HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY AGE GROUP
18.2.1.5. U.S. HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
18.2.1.6. U.S. HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY END USER
18.2.1.7. U.S. HER2 POSITIVE BREAST CANCER TREATMENT MARKET, BY DISTRIBUTION CHANNEL
18.2.1.8. CANADA
18.2.1.9. MEXICO
18.2.2 EUROPE
18.2.2.1. GERMANY
18.2.2.2. FRANCE
18.2.2.3. U.K.
18.2.2.4. ITALY
18.2.2.5. SPAIN
18.2.2.6. RUSSIA
18.2.2.7. TURKEY
18.2.2.8. BELGIUM
18.2.2.9. DENMARK
18.2.2.10. SWEDEN
18.2.2.11. POLAND
18.2.2.12. NORWAY
18.2.2.13. FINLAND
18.2.2.14. NETHERLANDS
18.2.2.15. SWITZERLAND
18.2.2.16. REST OF EUROPE
18.2.3 ASIA-PACIFIC
18.2.3.1. JAPAN
18.2.3.2. CHINA
18.2.3.3. SOUTH KOREA
18.2.3.4. INDIA
18.2.3.5. AUSTRALIA
18.2.3.6. SINGAPORE
18.2.3.7. THAILAND
18.2.3.8. MALAYSIA
18.2.3.9. INDONESIA
18.2.3.10. PHILIPPINES
18.2.3.11. TAIWAN
18.2.3.12. INDONESIA
18.2.3.13. VIETNAM
18.2.3.14. REST OF ASIA-PACIFIC
18.2.4 SOUTH AMERICA
18.2.4.1. BRAZIL
18.2.4.2. ARGENTINA
18.2.4.3. REST OF SOUTH AMERICA
18.2.5 MIDDLE EAST AND AFRICA
18.2.5.1. SOUTH AFRICA
18.2.5.2. SAUDI ARABIA
18.2.5.3. UAE
18.2.5.4. EGYPT
18.2.5.5. BAHRAIN
18.2.5.6. KUWAIT
18.2.5.7. QATAR
18.2.5.8. OMAN
18.2.5.9. ISRAEL
18.2.5.10. REST OF MIDDLE EAST AND AFRICA
19 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, SWOT AND DBMR ANALYSIS
20 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, COMPANY LANDSCAPE
20.1 COMPANY SHARE ANALYSIS: GLOBAL
20.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
20.3 COMPANY SHARE ANALYSIS: EUROPE
20.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
20.5 MERGERS & ACQUISITIONS
20.6 NEW PRODUCT DEVELOPMENT & APPROVALS
20.7 EXPANSIONS
20.8 REGULATORY CHANGES
20.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
21 GLOBAL HER2 POSITIVE BREAST CANCER TREATMENT MARKET, COMPANY PROFILE
21.1 ASTRAZENCA
21.1.1 COMPANY OVERVIEW
21.1.2 REVENUE ANALYSIS
21.1.3 GEOGRAPHIC PRESENCE
21.1.4 PRODUCT PORTFOLIO
21.1.5 RECENT DEVELOPMENTS
21.2 DAIICHI SANKYO
21.2.1 COMPANY OVERVIEW
21.2.2 REVENUE ANALYSIS
21.2.3 GEOGRAPHIC PRESENCE
21.2.4 PRODUCT PORTFOLIO
21.2.5 RECENT DEVELOPMENTS
21.3 BRISTOL MEYERS SQUIBB
21.3.1 COMPANY OVERVIEW
21.3.2 REVENUE ANALYSIS
21.3.3 GEOGRAPHIC PRESENCE
21.3.4 PRODUCT PORTFOLIO
21.3.5 RECENT DEVELOPMENTS
21.4 EISAI INC
21.4.1 COMPANY OVERVIEW
21.4.2 REVENUE ANALYSIS
21.4.3 GEOGRAPHIC PRESENCE
21.4.4 PRODUCT PORTFOLIO
21.4.5 RECENT DEVELOPMENTS
21.5 F-HOFFMANN LA ROCHE
21.5.1 COMPANY OVERVIEW
21.5.2 REVENUE ANALYSIS
21.5.3 GEOGRAPHIC PRESENCE
21.5.4 PRODUCT PORTFOLIO
21.5.5 RECENT DEVELOPMENTS
21.6 GSK
21.6.1 COMPANY OVERVIEW
21.6.2 REVENUE ANALYSIS
21.6.3 GEOGRAPHIC PRESENCE
21.6.4 PRODUCT PORTFOLIO
21.6.5 RECENT DEVELOPMENTS
21.7 GENETECH
21.7.1 COMPANY OVERVIEW
21.7.2 REVENUE ANALYSIS
21.7.3 GEOGRAPHIC PRESENCE
21.7.4 PRODUCT PORTFOLIO
21.7.5 RECENT DEVELOPMENTS
21.8 PFIZER
21.8.1 COMPANY OVERVIEW
21.8.2 REVENUE ANALYSIS
21.8.3 GEOGRAPHIC PRESENCE
21.8.4 PRODUCT PORTFOLIO
21.8.5 RECENT DEVELOPMENTS
21.9 MERCK & CO, INC
21.9.1 COMPANY OVERVIEW
21.9.2 REVENUE ANALYSIS
21.9.3 GEOGRAPHIC PRESENCE
21.9.4 PRODUCT PORTFOLIO
21.9.5 RECENT DEVELOPMENTS
21.1 PUMA BIOTECHNOLOGY INC
21.10.1 COMPANY OVERVIEW
21.10.2 REVENUE ANALYSIS
21.10.3 GEOGRAPHIC PRESENCE
21.10.4 PRODUCT PORTFOLIO
21.10.5 RECENT DEVELOPMENTS
21.11 ELI LILLY
21.11.1 COMPANY OVERVIEW
21.11.2 REVENUE ANALYSIS
21.11.3 GEOGRAPHIC PRESENCE
21.11.4 PRODUCT PORTFOLIO
21.11.5 RECENT DEVELOPMENTS
21.12 CELLTRION
21.12.1 COMPANY OVERVIEW
21.12.2 REVENUE ANALYSIS
21.12.3 GEOGRAPHIC PRESENCE
21.12.4 PRODUCT PORTFOLIO
21.12.5 RECENT DEVELOPMENTS
21.13 GILEAD SVIENCES
21.13.1 COMPANY OVERVIEW
21.13.2 REVENUE ANALYSIS
21.13.3 GEOGRAPHIC PRESENCE
21.13.4 PRODUCT PORTFOLIO
21.13.5 RECENT DEVELOPMENTS
21.14 AMGEN
21.14.1 COMPANY OVERVIEW
21.14.2 REVENUE ANALYSIS
21.14.3 GEOGRAPHIC PRESENCE
21.14.4 PRODUCT PORTFOLIO
21.14.5 RECENT DEVELOPMENTS
21.15 DR REDDY LABORATORIES
21.15.1 COMPANY OVERVIEW
21.15.2 REVENUE ANALYSIS
21.15.3 GEOGRAPHIC PRESENCE
21.15.4 PRODUCT PORTFOLIO
21.15.5 RECENT DEVELOPMENTS
21.16 MYLAN PHARMACEUTICALS
21.16.1 COMPANY OVERVIEW
21.16.2 REVENUE ANALYSIS
21.16.3 GEOGRAPHIC PRESENCE
21.16.4 PRODUCT PORTFOLIO
21.16.5 RECENT DEVELOPMENTS
21.17 KYOWA KRIN INTERNATIONAL
21.17.1 COMPANY OVERVIEW
21.17.2 REVENUE ANALYSIS
21.17.3 GEOGRAPHIC PRESENCE
21.17.4 PRODUCT PORTFOLIO
21.17.5 RECENT DEVELOPMENTS
21.18 JOHNSONS & JOHNSON (JANSSEN PHARMACEUTICASL)
21.18.1 COMPANY OVERVIEW
21.18.2 REVENUE ANALYSIS
21.18.3 GEOGRAPHIC PRESENCE
21.18.4 PRODUCT PORTFOLIO
21.18.5 RECENT DEVELOPMENTS
21.19 BAXTER HEALTHCARE
21.19.1 COMPANY OVERVIEW
21.19.2 REVENUE ANALYSIS
21.19.3 GEOGRAPHIC PRESENCE
21.19.4 PRODUCT PORTFOLIO
21.19.5 RECENT DEVELOPMENTS
21.2 AMNEAL PHARMACEUTICALS
21.20.1 COMPANY OVERVIEW
21.20.2 REVENUE ANALYSIS
21.20.3 GEOGRAPHIC PRESENCE
21.20.4 PRODUCT PORTFOLIO
21.20.5 RECENT DEVELOPMENTS
21.21 ABBOTT LABORATORIES
21.21.1 COMPANY OVERVIEW
21.21.2 REVENUE ANALYSIS
21.21.3 GEOGRAPHIC PRESENCE
21.21.4 PRODUCT PORTFOLIO
21.21.5 RECENT DEVELOPMENTS
21.22 ABBVIE INC
21.22.1 COMPANY OVERVIEW
21.22.2 REVENUE ANALYSIS
21.22.3 GEOGRAPHIC PRESENCE
21.22.4 PRODUCT PORTFOLIO
21.22.5 RECENT DEVELOPMENTS
21.23 NOVARTIS AG
21.23.1 COMPANY OVERVIEW
21.23.2 REVENUE ANALYSIS
21.23.3 GEOGRAPHIC PRESENCE
21.23.4 PRODUCT PORTFOLIO
21.23.5 RECENT DEVELOPMENTS
21.24 TEVA PHARMACEUTICALS
21.24.1 COMPANY OVERVIEW
21.24.2 REVENUE ANALYSIS
21.24.3 GEOGRAPHIC PRESENCE
21.24.4 PRODUCT PORTFOLIO
21.24.5 RECENT DEVELOPMENTS
21.25 APOTEX CORPORATION
21.25.1 COMPANY OVERVIEW
21.25.2 REVENUE ANALYSIS
21.25.3 GEOGRAPHIC PRESENCE
21.25.4 PRODUCT PORTFOLIO
21.25.5 RECENT DEVELOPMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
22 RELATED REPORTS
23 CONCLUSION
24 QUESTIONNAIRE
25 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












