Global Histiocytic Lymphoma Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
650.00 Million
USD
1,005.13 Million
2025
2033
USD
650.00 Million
USD
1,005.13 Million
2025
2033
| 2026 –2033 | |
| USD 650.00 Million | |
| USD 1,005.13 Million | |
|
|
|
|
Histiocytic Lymphoma Treatment Market Size
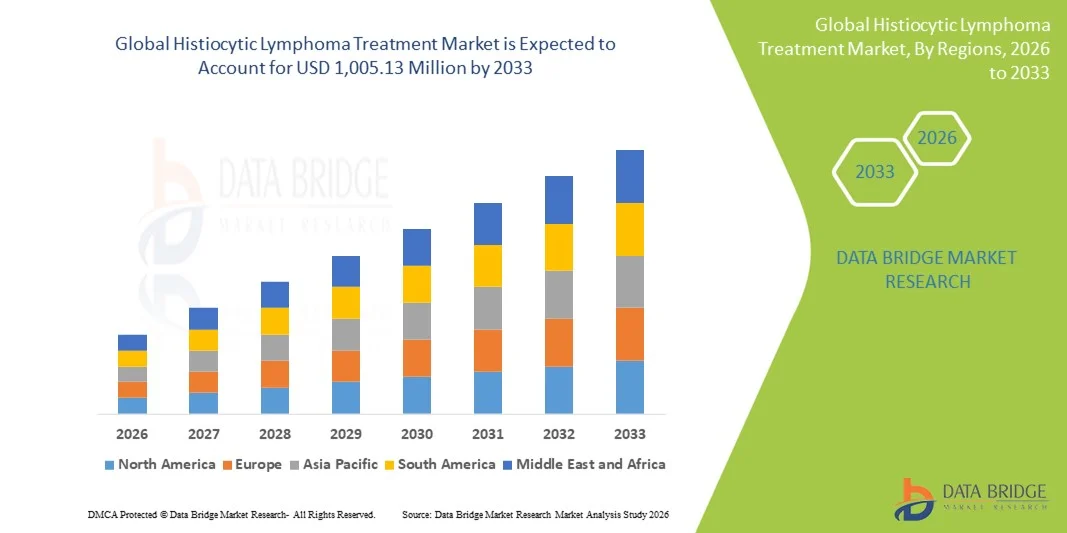
- The global histiocytic lymphoma treatment market size was valued at USD 650.00 million in 2025 and is expected to reach USD 1,005.13 million by 2033, at a CAGR of 5.60% during the forecast period
- The market growth is largely fueled by advancements in targeted therapies, immunotherapies, and precision medicine, improving diagnosis and treatment outcomes for rare histiocytic conditions including histiocytic lymphoma, which is driving higher adoption of specialized treatment protocols and investment in orphan oncology drug development
- Furthermore, rising awareness of rare cancers, expanded healthcare access, and increased R&D collaboration among pharmaceutical companies and research institutions are establishing histiocytic lymphoma treatment as a critical niche within the broader oncology space. These converging factors are accelerating the uptake of innovative therapies, thereby significantly boosting the industry’s growth
Histiocytic Lymphoma Treatment Market Analysis
- Histiocytic lymphoma treatments, including chemotherapy, targeted therapy, and immunotherapy, are increasingly vital in managing rare hematologic malignancies due to their ability to improve survival outcomes, reduce relapse rates, and offer personalized treatment approaches for patients with histiocytic disorders
- The escalating demand for histiocytic lymphoma treatments is primarily fueled by advancements in targeted therapies and immunotherapies, increased disease awareness, and expanding healthcare infrastructure, allowing earlier diagnosis and wider access to specialized treatment protocols
- North America dominated the histiocytic lymphoma treatment market with the largest revenue share of 47.2% in 2025 driven by well-established oncology healthcare infrastructure, high adoption of advanced therapies, robust R&D pipelines, and strong presence of key pharmaceutical companies developing novel histiocytic lymphoma treatments
- Asia-Pacific is expected to be the fastest-growing region in the histiocytic lymphoma treatment market during the forecast period, due to rising healthcare expenditure, improved access to oncology care, growing awareness of rare cancers, and increasing availability of advanced treatment options in emerging economies
- Targeted drug therapy segment dominated the histiocytic lymphoma treatment market with a market share of 43.9% in 2025 driven by its efficacy, reduced side-effect profile compared to conventional chemotherapy, and increasing approval of new targeted drugs for rare hematologic cancers
Report Scope and Histiocytic Lymphoma Treatment Market Segmentation
|
Attributes |
Histiocytic Lymphoma Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Histiocytic Lymphoma Treatment Market Trends
Advancements in Targeted and Immunotherapy Treatments
- A significant and accelerating trend in the global histiocytic lymphoma treatment market is the increasing adoption of targeted therapies and immunotherapies, which offer improved efficacy and reduced side effects compared to traditional chemotherapy
- Integration of precision medicine approaches, including genomic profiling and molecular diagnostics, enables clinicians to tailor treatments to individual patient profiles, enhancing treatment outcomes and reducing relapse rates
- The growing combination of immunotherapy with targeted therapy is redefining standard care protocols, offering patients more durable responses and fewer adverse effects compared to conventional treatments
- Emerging therapies such as bispecific antibodies and antibody-drug conjugates (ADCs) are opening new avenues for difficult-to-treat histiocytic lymphoma subtypes
- Digital health tools, including remote patient monitoring and AI-based treatment decision support, are improving therapy adherence and outcomes
- Collaborations between biotech companies and research institutions are accelerating clinical trials and the development of novel therapies
- Increasing investment in orphan drug development is attracting new entrants to the market, boosting competitive innovation
- For instance, next-generation sequencing (NGS) panels are increasingly used to identify actionable mutations in histiocytic lymphoma patients, guiding therapy selection
Histiocytic Lymphoma Treatment Market Dynamics
Driver
Rising Demand Due to Unmet Treatment Needs and Rare Cancer Awareness
- The increasing prevalence of rare hematologic cancers, coupled with growing awareness of histiocytic lymphoma, is a significant driver for the heightened demand for advanced therapies
- As patients and healthcare providers seek more effective and personalized treatment options, therapies such as immunotherapy and precision-targeted drugs are becoming preferred over conventional chemotherapy
- Furthermore, expanding healthcare infrastructure and oncology expertise in emerging markets are enabling broader access to advanced histiocytic lymphoma treatments
- Increasing R&D investments by pharmaceutical companies in rare hematologic malignancies are driving the development of innovative therapies with better efficacy and safety profiles
- Growing collaborations between pharma companies and academic research centers accelerate therapy availability and clinical adoption
- Rising awareness campaigns and patient advocacy initiatives are educating patients and healthcare providers about early diagnosis and treatment options
- Government incentives for orphan drug development and rare disease treatment are supporting market expansion
- For instance, CAR-T cell therapy is being explored for refractory cases, offering a potential lifesaving alternative where other treatments fail
Restraint/Challenge
High Treatment Costs and Limited Awareness in Emerging Markets
- The high cost of advanced therapies, including targeted and immunotherapy drugs, poses a significant challenge to widespread adoption, particularly in price-sensitive regions
- Limited disease awareness among healthcare providers and patients in certain regions results in delayed diagnosis and treatment initiation, constraining market growth
- Furthermore, stringent regulatory requirements for approval of orphan drugs and novel therapies create additional entry barriers for pharmaceutical companies
- Shortage of trained oncology specialists and limited advanced diagnostic infrastructure in certain regions can hinder timely treatment delivery
- Complex side-effect management for immunotherapies and targeted therapies can limit patient eligibility and adoption
- Supply chain challenges, including drug availability and cold-chain requirements for biologics, can restrict access in some regions
- High cost of continuous monitoring and follow-up treatments increases the overall economic burden on healthcare systems
- For instance, patients in rural areas may face delays in accessing molecular testing or targeted therapies due to insufficient local healthcare facilities
Histiocytic Lymphoma Treatment Market Scope
The market is segmented on the basis of treatment, cell type, diagnosis, end-users, and distribution channel.
- By Treatment
On the basis of treatment, the market is segmented into chemotherapy, radiation therapy, targeted drug therapy, chimeric antigen receptor (Car)-T cell therapy, bone marrow transplant, and immunotherapy. Targeted Drug Therapy dominated the market with the largest revenue share of 43.9% in 2025, driven by its higher efficacy and lower side effects compared to conventional chemotherapy. Patients and clinicians increasingly prefer targeted therapies due to their ability to selectively attack cancerous cells while sparing healthy tissue, improving treatment outcomes. The adoption is also supported by advancements in genomic profiling, which allows physicians to personalize treatment regimens based on actionable mutations. Pharmaceutical companies are investing heavily in R&D and clinical trials to develop novel targeted drugs for rare histiocytic lymphoma subtypes. Moreover, targeted therapies often show better patient compliance due to oral administration options, reducing hospital visits. Healthcare providers are increasingly integrating these therapies into standard care protocols, further consolidating market dominance.
CAR-T Cell Therapy is anticipated to witness the fastest growth from 2026 to 2033, driven by its revolutionary approach in treating refractory and relapsed histiocytic lymphoma. CAR-T therapy harnesses a patient’s own immune system to specifically target cancer cells, offering durable and potentially curative outcomes. The therapy’s growing adoption is fueled by increasing approvals for rare cancer indications and rising investments in advanced cell therapy manufacturing infrastructure. In addition, hospitals and specialty clinics are expanding access to CAR-T centers, enabling wider patient reach. Strategic partnerships between biotech firms and academic hospitals are accelerating clinical trials, further supporting growth. The increasing awareness of this therapy among oncologists and patients, coupled with promising clinical results, is encouraging rapid market expansion.
- By Cell Type
On the basis of cell type, the market is segmented into B-cell and T-cell. B-cell dominated the market in 2025, as most histiocytic lymphoma cases arise from B-cell lineage, driving higher demand for therapies specifically targeting this cell type. Approved treatments for B-cell histiocytic lymphoma, including monoclonal antibodies and targeted inhibitors, are widely available and have shown significant efficacy in clinical practice. The market dominance is reinforced by strong R&D focus and ongoing clinical trials for next-generation B-cell therapies. Furthermore, healthcare providers prefer B-cell-specific therapies due to their predictability in patient response and manageable side-effect profiles. Patient awareness campaigns and educational initiatives have also increased early detection and treatment adoption for B-cell histiocytic lymphoma. The availability of companion diagnostic tests enhances treatment personalization and optimizes outcomes.
T-cell is expected to witness the fastest growth from 2026 to 2033, as novel therapies targeting T-cell lymphomas are emerging with significant clinical success. T-cell histiocytic lymphoma is rarer and has historically lacked effective therapies, creating high growth potential for innovative treatment approaches. Advancements in immunotherapy, such as CAR-T and bispecific antibodies targeting T-cell antigens, are accelerating adoption. Expanding clinical trials and regulatory approvals are facilitating market penetration in developed and emerging countries. Increased physician awareness and patient advocacy programs are also contributing to faster uptake. The need for therapies with better survival outcomes and reduced toxicity drives this segment’s rapid expansion.
- By Diagnosis
On the basis of diagnosis, the market is segmented into Lymph Node Biopsy, CT Scan, PET Scan, Ultrasound, and Chest X-ray. Lymph Node Biopsy dominated the market with the largest revenue share in 2025, as it remains the gold standard for confirming histiocytic lymphoma diagnosis and differentiating it from other hematologic malignancies. The procedure allows for detailed histopathological and immunophenotypic analysis, which guides treatment selection. Healthcare providers rely on biopsy results for accurate staging and therapy planning, making it essential for clinical decision-making. Moreover, increasing access to advanced pathology labs has improved biopsy utilization in developed and emerging markets. Patient outcomes are significantly improved through precise diagnosis, further reinforcing market dominance. In addition, the integration of molecular testing with biopsy samples enables identification of actionable mutations, supporting targeted therapy administration.
PET Scan is expected to witness the fastest growth from 2026 to 2033, driven by its ability to provide whole-body imaging and detect early disease involvement with high accuracy. PET scans are increasingly used to monitor treatment response and detect relapse in histiocytic lymphoma patients. Advancements in PET imaging technology, including hybrid PET/CT systems, are enhancing diagnostic precision and patient convenience. Rising adoption in oncology centers and improved insurance coverage in developed countries support rapid market growth. The non-invasive nature and high sensitivity of PET imaging encourage physicians to incorporate it into routine care. Increasing awareness among clinicians about the value of PET scans in treatment planning is further accelerating adoption.
- By End-Users
On the basis of end-users, the market is segmented into clinic, hospital, and others. Hospitals dominated the market in 2025, as they offer comprehensive oncology care, including advanced therapies, diagnostic services, and specialized treatment centers for rare cancers. The availability of multidisciplinary teams and infrastructure for complex treatments such as CAR-T therapy and bone marrow transplant supports hospital dominance. Hospitals also have established referral networks and access to clinical trials, ensuring patients receive cutting-edge treatments. Government support and insurance coverage for hospital-based therapies further enhance market share. In addition, hospitals can provide integrated care, including diagnostics, treatment, and follow-up, which is critical for histiocytic lymphoma management. High patient trust in hospital care also contributes to the segment’s market leadership.
Clinics are expected to witness the fastest growth from 2026 to 2033, driven by increasing availability of outpatient oncology services and targeted therapy administration in specialized clinics. Clinics offer convenience, personalized care, and lower costs for certain therapies compared to hospitals. Expansion of clinic networks in urban and semi-urban regions enhances patient access to advanced treatments. Partnerships with pharmaceutical companies for therapy delivery and patient monitoring are facilitating adoption. Clinics are also leveraging telemedicine and digital health platforms to support treatment adherence and follow-up care. The growing acceptance of outpatient therapy models in developed and emerging markets contributes to this segment’s rapid growth.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. Hospital Pharmacy dominated the market in 2025, as most advanced therapies for histiocytic lymphoma, including CAR-T and targeted drugs, are administered under hospital supervision. Hospital pharmacies ensure proper storage, dosing, and handling of complex biologics, which is critical for patient safety. The presence of trained pharmacists and oncology care teams facilitates compliance with treatment protocols. Hospitals also provide integrated access to diagnostics, treatment, and monitoring, strengthening hospital pharmacy market dominance. Government and insurance reimbursement policies often favor hospital-dispensed therapies, supporting adoption. In addition, hospitals are preferred by patients for therapies requiring close medical supervision, such as CAR-T cell infusions.
Online Pharmacy is expected to witness the fastest growth from 2026 to 2033, fueled by rising e-commerce adoption, improved digital infrastructure, and increasing patient preference for home delivery of oral targeted therapies. Online pharmacies offer convenience, accessibility, and competitive pricing, making them attractive to patients in both urban and remote regions. Integration with telemedicine platforms enables prescription verification, adherence tracking, and patient counseling. Online channels also reduce the burden on hospital pharmacies, especially for chronic oral therapies. Increasing regulatory approvals and secure logistics solutions are further boosting market penetration. Growing awareness of online pharmacy options among patients and caregivers accelerates adoption, particularly in developed markets
Histiocytic Lymphoma Treatment Market Regional Analysis
- North America dominated the histiocytic lymphoma treatment market with the largest revenue share of 47.2% in 2025 driven by well-established oncology healthcare infrastructure, high adoption of advanced therapies, robust R&D pipelines, and strong presence of key pharmaceutical companies developing novel histiocytic lymphoma treatments
- Patients and healthcare providers in the region highly value the availability of targeted therapies, immunotherapies, and advanced diagnostic technologies, which enable accurate disease identification and personalized treatment planning, leading to improved clinical outcomes
- This widespread adoption is further supported by favorable reimbursement policies, strong pharmaceutical R&D activity, and a high concentration of specialized cancer centers, establishing North America as a leading market for histiocytic lymphoma treatment across both hospital-based and outpatient oncology settings
The U.S. Histiocytic Lymphoma Treatment Market Insight
The U.S. histiocytic lymphoma treatment market captured the largest revenue share within North America in 2025, fueled by advanced oncology infrastructure, high awareness of rare hematologic malignancies, and early adoption of innovative therapies. Healthcare providers in the U.S. increasingly prioritize precision medicine, including targeted therapies and immunotherapies, to improve patient outcomes. The strong presence of leading pharmaceutical companies and active clinical trial pipelines further propels market growth. Moreover, favorable reimbursement frameworks and access to specialized cancer centers significantly contribute to the expansion of histiocytic lymphoma treatment adoption across the country.
Europe Histiocytic Lymphoma Treatment Market Insight
The Europe histiocytic lymphoma treatment market is projected to expand at a notable CAGR during the forecast period, primarily driven by increasing awareness of rare cancers and improvements in diagnostic capabilities. Rising investments in oncology research, coupled with supportive regulatory frameworks for orphan drugs, are fostering treatment adoption across the region. European healthcare systems emphasize early diagnosis and evidence-based treatment protocols, supporting market growth. The region is witnessing increased utilization of targeted and immunotherapies across hospitals and specialized oncology centers, particularly in Western Europe.
U.K. Histiocytic Lymphoma Treatment Market Insight
The U.K. histiocytic lymphoma treatment market is anticipated to grow at a steady CAGR during the forecast period, driven by the country’s strong public healthcare system and growing focus on rare disease management. Increasing adoption of advanced diagnostic techniques and access to specialized oncology services are supporting treatment uptake. National health initiatives aimed at improving early cancer detection are also contributing to market growth. In addition, the U.K.’s active participation in clinical research and rare cancer trials is expected to further strengthen market development.
Germany Histiocytic Lymphoma Treatment Market Insight
The Germany histiocytic lymphoma treatment market is expected to expand at a considerable CAGR, supported by well-established healthcare infrastructure and strong emphasis on medical innovation. Germany’s leadership in pharmaceutical research and biotechnology facilitates access to advanced therapies for rare hematologic malignancies. The availability of specialized cancer centers and precision diagnostic tools enhances treatment accuracy and outcomes. Furthermore, comprehensive insurance coverage and a strong regulatory framework promote the adoption of novel therapies, reinforcing market growth across the country.
Asia-Pacific Histiocytic Lymphoma Treatment Market Insight
The Asia-Pacific histiocytic lymphoma treatment market is poised to grow at the fastest CAGR during the forecast period, driven by improving healthcare infrastructure, rising cancer awareness, and increasing access to advanced oncology treatments. Countries such as China, Japan, and India are investing heavily in cancer care capabilities, enabling wider diagnosis and treatment of rare lymphomas. Growing pharmaceutical manufacturing capacity and expanding clinical research activities are further supporting market expansion. In addition, government initiatives to strengthen oncology care systems are improving patient access to specialized treatments across the region.
Japan Histiocytic Lymphoma Treatment Market Insight
The Japan histiocytic lymphoma treatment market is gaining momentum due to the country’s advanced healthcare system, strong emphasis on early diagnosis, and growing adoption of targeted therapies. Japan’s focus on precision medicine and molecular diagnostics supports effective treatment planning for rare cancers. The increasing number of specialized oncology centers and integration of innovative therapies into clinical practice are fueling market growth. Moreover, Japan’s aging population is contributing to higher cancer incidence, further driving demand for effective lymphoma treatments.
India Histiocytic Lymphoma Treatment Market Insight
The India histiocytic lymphoma treatment market accounted for a significant share in Asia-Pacific in 2025, supported by expanding oncology infrastructure and increasing awareness of rare hematologic malignancies. India’s growing network of cancer hospitals and specialty clinics is improving access to advanced diagnostics and treatments. Rising healthcare expenditure and government initiatives aimed at strengthening cancer care are further propelling market growth. In addition, the availability of cost-effective therapies and the expansion of domestic pharmaceutical manufacturing are enhancing treatment accessibility across urban and semi-urban regions.
Histiocytic Lymphoma Treatment Market Share
The Histiocytic Lymphoma Treatment industry is primarily led by well-established companies, including:
- Novartis AG (Switzerland)
- Gilead Sciences, Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Pfizer Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- AstraZeneca (U.K.)
- BeiGene, Ltd. (U.S.)
- Cellectis (France)
- Mustang Bio, Inc. (U.S.)
- BioInvent International AB (Sweden)
- Seagen Inc. (U.S.)
- Autolus Therapeutics plc (U.K.)
- Allogene Therapeutics, Inc. (U.S.)
- CARsgen Therapeutics Holdings Limited (China)
- Atara Biotherapeutics, Inc. (U.S.)
- Medigene AG (Germany)
What are the Recent Developments in Global Histiocytic Lymphoma Treatment Market?
- In November 2025, the FDA granted traditional approval to epcoritamab-bysp in combination with lenalidomide and rituximab for relapsed or refractory follicular lymphoma following positive results from the phase 3 EPCORE FL-1 trial, representing a significant new standard of care for this patient population
- In May 2025, researchers at the University of Pennsylvania reported promising results from a phase I study of a next-generation “armored” CAR T cell therapy for relapsed/refractory lymphoma that showed an 81% reduction in cancer burden and a 52% complete remission rate, highlighting advancements in CAR T design and efficacy
- In March 2024, the U.S. FDA granted accelerated approval to the CAR-T cell therapy Breyanzi (lisocabtagene maraleucel) for adult patients with relapsed or refractory follicular lymphoma who have received at least two prior systemic therapies, expanding CAR-T use in rare lymphomas. This approval marked a major advancement in immunotherapy for difficult-to-treat B-cell lymphomas, offering a one-time cellular therapy option with durable responses for patients with limited alternatives
- In July 2024, a novel CAR-T cell therapy targeting an alternative antigen showed a high rate of durable complete responses in patients with relapsed or refractory large B-cell lymphoma in a Phase 1 trial, leading to an FDA Breakthrough Therapy designation to expedite its development. This represents a promising innovation for patients whose cancers have stopped responding to existing CAR-T therapies and highlights ongoing efforts to improve outcomes in aggressive lymphoma subtypes
- In October 2022, the U.S. Food and Drug Administration (FDA) approved the oral MEK inhibitor cobimetinib (Cotellic®) for the treatment of adults with histiocytic neoplasms, including Erdheim-Chester disease, Rosai-Dorfman disease, and Langerhans cell histiocytosis, providing a new targeted therapy option for rare histiocytic disorders
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.