Global Hurler Scheie Syndrome Market
Market Size in USD Million
CAGR :
% 
 USD
530.50 Million
USD
693.18 Million
2024
2032
USD
530.50 Million
USD
693.18 Million
2024
2032
| 2025 –2032 | |
| USD 530.50 Million | |
| USD 693.18 Million | |
|
|
|
|
Hurler-Scheie Syndrome Market Size
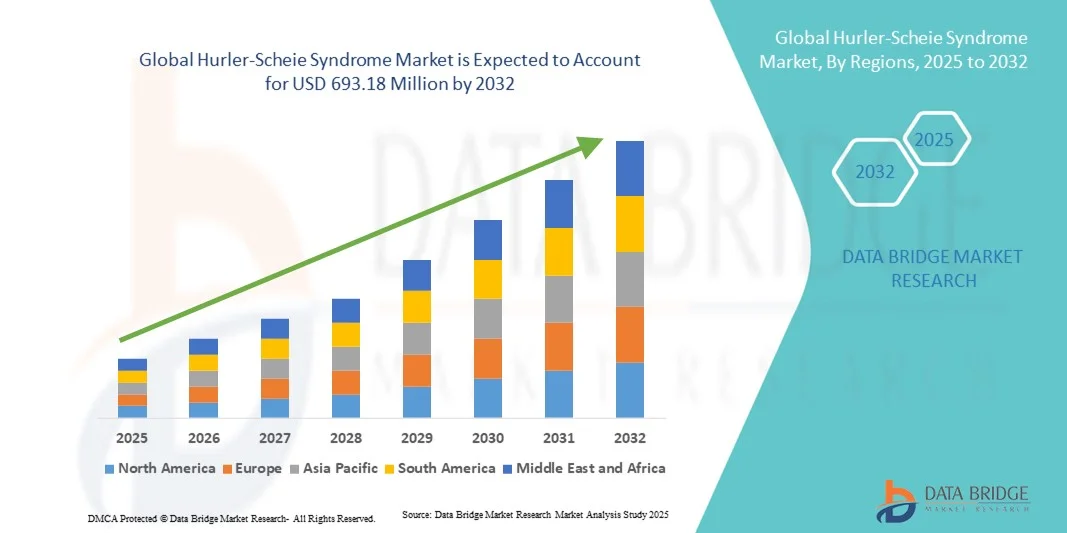
- The global Hurler-Scheie Syndrome market size was valued at USD 530.50 million in 2024 and is expected to reach USD 693.18 million by 2032, at a CAGR of 3.40% during the forecast period
- The market growth is primarily driven by increasing awareness of lysosomal storage disorders, advancements in enzyme replacement therapies (ERT), and improved diagnostic capabilities enabling early identification and intervention for patients with Hurler-Scheie syndrome
- Furthermore, expanding research efforts in gene therapy and precision medicine, along with supportive regulatory and reimbursement frameworks, are enhancing treatment accessibility and innovation, thereby fueling the global Hurler-Scheie Syndrome market growth throughout the forecast period
Hurler-Scheie Syndrome Market Analysis
- Hurler-Scheie Syndrome, a rare subtype of mucopolysaccharidosis type I (MPS I), is gaining prominence due to increased clinical awareness, advancements in enzyme replacement therapy (ERT) and gene therapy, and improved global access to diagnostic technologies facilitating early detection and intervention
- The market expansion is fueled by growing government support for rare disease research, favorable orphan drug regulations, and increased availability of molecular and biochemical testing for precise diagnosis and patient management
- North America dominated the Hurler-Scheie Syndrome market with the largest revenue share of 38% in 2024, driven by robust healthcare infrastructure, high treatment accessibility, and strong biopharmaceutical R&D activity in rare genetic disorders
- Asia-Pacific is expected to be the fastest-growing region during the forecast period, attributed to rising healthcare investments, expanding diagnostic capabilities, and increasing awareness of lysosomal storage disorders across countries such as China, Japan, and India
- 1,9-Dimethylmethylene Blue (DMB) Test segment dominated the Hurler-Scheie Syndrome market with a market share of 44.1% in 2024, owing to its accuracy, affordability, and routine use in measuring glycosaminoglycan (GAG) levels for early detection and monitoring of MPS I, including Hurler-Scheie Syndrome
Report Scope and Hurler-Scheie Syndrome Market Segmentation
|
Attributes |
Hurler-Scheie Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Hurler-Scheie Syndrome Market Trends
Advancements in Gene Therapy and Enzyme Delivery Innovations
- A significant and accelerating trend in the global Hurler-Scheie Syndrome market is the rapid advancement of gene therapy and enzyme delivery technologies designed to address the root cause of α-L-iduronidase enzyme deficiency and improve long-term treatment efficacy
- For instance, REGENXBIO and Sarepta Therapeutics are developing adeno-associated virus (AAV)-based gene therapy platforms to deliver functional IDUA genes directly to target tissues, aiming for sustained therapeutic benefit. Similarly, companies such as Orchard Therapeutics are focusing on hematopoietic stem cell gene therapy to achieve durable enzyme production
- Gene therapy advancements are complemented by innovations in enzyme replacement therapy (ERT), including extended half-life formulations and novel delivery routes such as intrathecal administration to cross the blood–brain barrier for neurological symptom management. For instance, clinical programs are exploring next-generation ERTs with enhanced bioavailability and targeted tissue distribution for improved outcomes
- The integration of advanced drug delivery systems and precision medicine approaches is reshaping the treatment landscape by offering more effective, patient-specific therapeutic options for Hurler-Scheie Syndrome
- This growing trend toward curative and long-lasting therapies is fundamentally transforming patient management strategies and attracting significant investment from biotech companies focusing on rare genetic disorders
- The demand for advanced gene therapy and targeted enzyme replacement solutions is expanding rapidly as patients and healthcare providers increasingly seek safer, more durable, and disease-modifying treatment options
Hurler-Scheie Syndrome Market Dynamics
Driver
Increasing Awareness, Early Diagnosis, and Supportive Regulatory Environment
- The growing global awareness of lysosomal storage disorders, coupled with advancements in diagnostic capabilities such as molecular and biochemical assays, is driving early diagnosis and timely intervention for Hurler-Scheie Syndrome
- For instance, in March 2024, the U.S. FDA granted Rare Pediatric Disease Designation to a novel gene therapy candidate for MPS I, encouraging accelerated research and development in this therapeutic domain. Such regulatory support and incentives are expected to drive the Hurler-Scheie Syndrome market growth in the forecast period
- As healthcare systems improve their genetic testing infrastructure, patients are increasingly identified at earlier stages, enabling better clinical outcomes through enzyme replacement and gene-based treatments
- Furthermore, favorable government policies, increased orphan drug approvals, and reimbursement support are creating a conducive environment for the commercialization of advanced therapies for Hurler-Scheie Syndrome
- The rise in patient advocacy initiatives and global research collaborations is also enhancing disease awareness and driving sustained investments into rare disease treatment innovation. The trend toward precision medicine and biomarker-based diagnosis further contributes to market expansion
Restraint/Challenge
High Treatment Costs and Limited Patient Accessibility
- The extremely high cost of enzyme replacement and gene therapies poses a significant challenge to market expansion, particularly in developing regions with limited healthcare funding for rare diseases
- For instance, treatment costs for lifelong ERT or single-dose gene therapy can exceed several hundred thousand dollars per patient annually, restricting access for many families despite clinical efficacy
- Addressing these affordability challenges through innovative pricing models, insurance coverage expansion, and global health partnerships is crucial for improving equitable treatment access. Companies such as Sanofi and BioMarin are actively exploring cost-sharing and compassionate use programs to support patient inclusion
- In addition, the scarcity of specialized treatment centers and trained clinicians for managing MPS I further limits timely diagnosis and consistent therapy delivery, especially in emerging economies
- While ongoing policy initiatives aim to improve healthcare access for rare disease patients, disparities in diagnostic infrastructure and treatment affordability continue to hinder global market penetration
- Overcoming these challenges through coordinated global health programs, expanded patient registries, and increased funding for rare disease treatment access will be essential for sustainable market growth
Hurler-Scheie Syndrome Market Scope
The market is segmented on the basis of diagnosis and symptoms.
- By Diagnosis
On the basis of diagnosis, the Hurler-Scheie Syndrome market is segmented into 1,9-dimethylmethylene blue (DMB) test, glycosaminoglycan (GAG) electrophoresis, and others. The 1,9-Dimethylmethylene Blue (DMB) Test segment dominated the Hurler-Scheie Syndrome market with the largest revenue share of 44.1% in 2024, owing to its widespread use in detecting elevated glycosaminoglycan (GAG) levels in urine samples an essential indicator of mucopolysaccharidosis (MPS) disorders. The test’s high sensitivity, affordability, and ease of implementation make it the preferred initial screening method in both developed and developing healthcare systems. In addition, the DMB test provides a rapid and cost-effective means for early detection, which is critical for timely initiation of enzyme replacement therapy (ERT) or gene therapy. Its non-invasive nature and compatibility with standard laboratory settings have further driven its adoption in clinical diagnostics. The expansion of newborn screening programs across North America and Europe is also expected to strengthen the demand for DMB testing, enhancing its dominance during the forecast period.
The Glycosaminoglycan (GAG) Electrophoresis segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by its ability to provide precise characterization and differentiation of specific GAG subtypes, including dermatan sulfate and heparan sulfate—biomarkers associated with Hurler-Scheie Syndrome. This advanced diagnostic approach enables confirmation of disease subtype following initial screening, thereby supporting personalized treatment decisions. The growing use of electrophoresis in combination with tandem mass spectrometry (MS/MS) and liquid chromatography (LC) for higher diagnostic accuracy is further accelerating segment growth. Increasing R&D investment in refining electrophoretic techniques for rare metabolic disorders and the rising adoption of confirmatory biochemical testing in specialized laboratories are boosting its clinical importance. Moreover, expanding healthcare infrastructure in Asia-Pacific is expected to enhance accessibility to these advanced diagnostic methods, contributing to its rapid market growth.
- By Symptoms
On the basis of symptoms, the Hurler-Scheie Syndrome market is segmented into abnormal heart valve morphology, abnormal vertebral morphology, abnormality of the tonsils, and others. The Abnormal Heart Valve Morphology segment dominated the Hurler-Scheie Syndrome market in 2024, as cardiac complications such as valvular thickening, regurgitation, and stenosis are among the most prevalent and clinically significant manifestations of the disorder. Early cardiac involvement often drives patients toward specialized treatment and monitoring, leading to a higher diagnostic and therapeutic demand in this category. Cardiologists increasingly recommend regular echocardiographic evaluations for patients diagnosed with MPS I disorders to prevent life-threatening cardiac events. The growing integration of multidisciplinary care, involving cardiology and genetics teams, enhances disease management and supports the dominance of this segment. Furthermore, improvements in non-invasive cardiac imaging and access to advanced care centers are facilitating timely diagnosis and intervention for heart-related symptoms, reinforcing its market leadership.
The Abnormal Vertebral Morphology segment is projected to experience the fastest CAGR from 2025 to 2032, owing to increasing clinical recognition of skeletal deformities, including kyphosis, scoliosis, and vertebral dysplasia, as early diagnostic indicators of Hurler-Scheie Syndrome. Orthopedic complications significantly impact patients’ mobility and quality of life, driving the need for early musculoskeletal evaluation and corrective intervention. The rising availability of advanced imaging modalities such as MRI and 3D CT scans has improved the precision of vertebral assessments, supporting better clinical outcomes. For instance, early spinal evaluation can guide timely orthopedic or surgical interventions, minimizing long-term disability. In addition, increased clinical research into skeletal manifestations of lysosomal storage disorders and the inclusion of bone and joint parameters in clinical trial endpoints are strengthening the growth of this segment globally.
Hurler-Scheie Syndrome Market Regional Analysis
- North America dominated the Hurler-Scheie Syndrome market with the largest revenue share of 38% in 2024, driven by robust healthcare infrastructure, high treatment accessibility, and strong biopharmaceutical R&D activity in rare genetic disorders
- Patients and healthcare providers in the region benefit from widespread access to enzyme replacement therapy (ERT), genetic testing, and gene therapy clinical trials, supported by favorable reimbursement frameworks and regulatory incentives for orphan drugs
- This strong regional performance is further reinforced by high disease awareness, collaborative research between academic and clinical institutions, and expanding newborn screening programs, establishing North America as a key hub for innovation and advanced care in Hurler-Scheie Syndrome management
U.S. Hurler-Scheie Syndrome Market Insight
The U.S. Hurler-Scheie Syndrome market captured the largest revenue share of over 78% in 2024 within North America, driven by the presence of advanced healthcare infrastructure, strong clinical research activity, and early adoption of enzyme replacement and gene therapies. Growing awareness of lysosomal storage disorders and extensive newborn screening programs are improving early diagnosis rates. The U.S. market is further fueled by the availability of orphan drug incentives, robust funding for rare disease research, and active participation of major pharmaceutical and biotechnology companies. Moreover, collaborations between academic institutions and patient advocacy groups continue to support clinical trials, accelerating innovation and treatment accessibility.
Europe Hurler-Scheie Syndrome Market Insight
The Europe Hurler-Scheie Syndrome market is projected to grow at a substantial CAGR throughout the forecast period, primarily driven by favorable regulatory frameworks such as the EU Orphan Drug Regulation and the expansion of national rare disease networks. Increasing government support for early genetic screening and growing collaboration between European research institutions are fostering clinical advancements. European countries are witnessing increased awareness of lysosomal storage disorders, leading to higher diagnosis and treatment rates. Furthermore, healthcare systems across Western Europe are integrating advanced enzyme replacement and gene therapy programs, ensuring broader patient access to innovative treatments.
U.K. Hurler-Scheie Syndrome Market Insight
The U.K. Hurler-Scheie Syndrome market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by a strong emphasis on rare disease research and the expansion of genomic testing under the National Health Service (NHS). Increasing patient identification through national registries and newborn screening initiatives supports early intervention and effective therapy management. The presence of leading research centers focusing on lysosomal storage disorders further strengthens the market. In addition, favorable reimbursement policies and government support for orphan drug development are expected to sustain long-term growth in the U.K. market.
Germany Hurler-Scheie Syndrome Market Insight
The Germany Hurler-Scheie Syndrome market is expected to expand at a considerable CAGR during the forecast period, fueled by the country’s advanced biotechnology ecosystem and strong focus on translational research in genetic and metabolic disorders. High awareness among healthcare providers and the availability of comprehensive diagnostic services contribute to early disease detection. Germany’s commitment to innovation and healthcare sustainability also supports investment in advanced therapies, including gene and enzyme replacement treatments. Furthermore, increasing patient participation in clinical trials and partnerships between hospitals and research institutions are driving continuous progress in the management of Hurler-Scheie Syndrome.
Asia-Pacific Hurler-Scheie Syndrome Market Insight
The Asia-Pacific Hurler-Scheie Syndrome market is poised to grow at the fastest CAGR of 23.6% from 2025 to 2032, driven by increasing healthcare expenditure, improved diagnostic infrastructure, and rising awareness of rare genetic diseases in countries such as China, Japan, and India. The growing implementation of national newborn screening programs and expansion of rare disease policies are facilitating earlier diagnosis and treatment access. Furthermore, APAC’s emergence as a biotechnology and clinical research hub is attracting investment from global pharmaceutical firms. Rising collaborations between local research institutions and international organizations are expected to accelerate therapeutic development and availability in the region.
Japan Hurler-Scheie Syndrome Market Insight
The Japan Hurler-Scheie Syndrome market is gaining momentum due to the nation’s advanced medical technology, strong regulatory support for orphan drugs, and well-established rare disease research infrastructure. The Japanese government’s active promotion of early diagnosis through national screening initiatives is enhancing patient identification and care. The increasing number of clinical studies focusing on enzyme and gene therapy solutions for MPS disorders further fuels market growth. Moreover, Japan’s highly developed healthcare system and emphasis on innovative, patient-centric therapies are expected to strengthen long-term market expansion.
India Hurler-Scheie Syndrome Market Insight
The India Hurler-Scheie Syndrome market accounted for the largest market revenue share within Asia-Pacific in 2024, supported by the country’s expanding rare disease awareness, improving diagnostic capabilities, and growing healthcare infrastructure. India’s government-led rare disease policy and inclusion of lysosomal storage disorders under treatment support schemes are creating favorable conditions for patient access. In addition, the increasing role of domestic biotechnology companies in enzyme formulation development and international research collaborations are driving market growth. The combination of affordable diagnostic technologies, medical outreach programs, and rising public health investment continues to propel the Hurler-Scheie Syndrome market in India.
Hurler-Scheie Syndrome Market Share
The Hurler-Scheie Syndrome industry is primarily led by well-established companies, including:
- BioMarin (U.S.)
- Sanofi (France)
- Takeda Pharmaceutical Company Limited (Japan)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Orchard Therapeutics plc (U.K.)
- Denali Therapeutics Inc. (U.S.)
- Regenxbio Inc. (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- Abeona Therapeutics Inc. (U.S.)
- Sangamo Therapeutics, Inc. (U.S.)
- JCR Pharmaceuticals Co., Ltd. (Japan)
- LG Chem, Ltd. (South Korea)
- ArmaGen, Inc. (U.S.)
- Idorsia Pharmaceuticals Ltd (Switzerland)
- uniQure N.V. (Netherlands)
- Sigilon Therapeutics (U.S.)
- Orchard Bio (U.K.)
- Inventiva (France)
- Krystal Biotech, Inc. (U.S.)
- Rocket Pharmaceuticals, Inc. (U.S.)
What are the Recent Developments in Global Hurler-Scheie Syndrome Market?
- In July 2025, Orchard Therapeutics announced that the last patient was treated in its registrational trial of OTL-203, an autologous ex vivo gene-corrected hematopoietic stem cell therapy for MPS I (Hurler subtype). This marks a key milestone in advancing toward potential regulatory submission. OTL-203 has shown encouraging early results in improving skeletal, respiratory, and cognitive outcomes areas where conventional enzyme replacement and transplants often fall short
- In March 2025, REGENXBIO and Nippon Shinyaku finalized a strategic partnership to co-develop and commercialize the investigational gene therapy RGX-111 for Mucopolysaccharidosis Type I (MPS I), which includes Hurler and Hurler-Scheie subtypes, across the U.S. and Asian markets. This collaboration aims to accelerate clinical development, expand patient access, and strengthen RGX-111’s potential to address central nervous system (CNS) symptoms an area unmet by current enzyme replacement therapies
- In October 2024, UCSF Benioff Children’s Hospitals launched a novel gene therapy trial enrolling infants diagnosed with Hurler syndrome, seeking to determine whether gene therapy can surpass bone-marrow transplant the current standard of care in treating both systemic and neurological symptoms. The study investigates whether direct gene delivery early in life could prevent irreversible damage to the brain and other organs
- In September 2024, JCR Pharmaceuticals showcased data at the SSIEM 2024 Congress featuring its proprietary J-Brain Cargo® technology, an innovative blood-brain-barrier-penetrating AAV vector platform designed for enzyme replacement therapy in MPS I and other lysosomal storage disorders. This technology aims to deliver therapeutic enzymes directly to the central nervous system, addressing one of the major limitations of existing treatments
- In May 2024, researchers published findings in Science Translational Medicine showing that a gene therapy administered to toddlers with Hurler syndrome not only corrected enzyme deficiencies but also improved growth, skeletal development, and joint flexibility effects rarely achieved by standard enzyme replacement therapy. The study demonstrated durable enzyme activity and measurable physical improvements, offering hope for addressing musculoskeletal complications that persist even after early interventions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.